Professional Documents
Culture Documents
Ammonia Mind Map
Ammonia Mind Map
Uploaded by
Peer MohamedOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ammonia Mind Map
Ammonia Mind Map
Uploaded by
Peer MohamedCopyright:
Available Formats
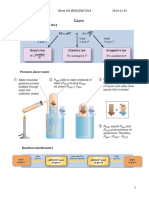
It can go both forward
and backward at the
same time
It is a chemical reaction
in which the conversion
Hydrogen gas (from the of reactants to products
What is a reversible
cracking of petroleum) and converting
reaction?
products to reactants
co-occur.
Nitrogen gas (from the
-The higher the fractional distillation of
pressure, the higher the liquid air N2 (g) + 3 H2 (g) ⇋ 2 NH3
yield of ammonia (high (g)
pressure also increases Examples of reversible
the speed of reaction) - How is the optimal reactions
However, maintaining pressure is selected? Ammonia NH4Cl (s) ⇋ NH3 (g) +
It is manufactured by
high pressure is costly HCl (g)
the Haber process
thus, there is a limit to
the amount of pressure
1. N2 and H2 are mixed
that can be applied
in the proportion of 1:3
4. A mixture of NH3, H2
by volume 2. The
and N2 is obtained and
-The lower the mixture of gases is
cooled 5. Ammonia gas
temperature, the Conditions: Pressure of compressed to 250 atm
condenses to form
higher the yield of 250 atm, Temperature 3. The gases are heated
Steps of the process liquid ammonia 6.
ammonia It is because, of 450°C, The presence to 450°C and passed
Unreacted N2 and H2
the decomposition of How is the optimal of an iron catalyst over finely divided iron,
are pumped back into
ammonia into H2 and temperature selected? since the reaction is
the converter for
N2 is reduced thus, a exothermic, only
further reaction
relatively high 10-15% of N2 and H2 is
temperature of 450°C is converted to NH3
used
-It is used to increase Why is an iron catalyst
the speed of reaction used?
When an ammonium The formation
salt is heated with an (displacement) of
alkali (eg NaOH, CaOH), ammonia from
ammonia is displaced ammonium salts and
from the salt alkalis
You might also like
- Haber Process FinalDocument31 pagesHaber Process FinalAdil Yaqub - 74665/TCHR/CNTBNo ratings yet
- EN 13889 - 2008 - ShacklesDocument4 pagesEN 13889 - 2008 - ShacklesRoby MastreNo ratings yet
- BS EN 10162 2003 Cold Rolled Steel Sections Technical Delivery Conditions Dimensions and Cross Sectional TolerancesDocument23 pagesBS EN 10162 2003 Cold Rolled Steel Sections Technical Delivery Conditions Dimensions and Cross Sectional TolerancesKader Boucenna100% (1)
- Process Simulation of Ammonia PlantDocument9 pagesProcess Simulation of Ammonia Planthamidrezaee008No ratings yet
- Rates of Reactions PDFDocument18 pagesRates of Reactions PDFLin Xian XingNo ratings yet
- Business Plan For Utstar Cement FactoryDocument8 pagesBusiness Plan For Utstar Cement Factoryravi818780% (5)
- Equation (G) Nitrogen: HydrogenDocument1 pageEquation (G) Nitrogen: HydrogenMUHAMMAD LUQMAN HAKIMI MOHD ZAMRINo ratings yet
- Chapter 2 - Compressible FlowDocument58 pagesChapter 2 - Compressible FlowLayike AlemayehuNo ratings yet
- Challenges in Kinetic Modeling of Ammonia Pyrolysis - 2022 - Fuel CommunicationsDocument12 pagesChallenges in Kinetic Modeling of Ammonia Pyrolysis - 2022 - Fuel CommunicationsHa Di KhNo ratings yet
- Energies 16 05072Document19 pagesEnergies 16 05072nuli chanNo ratings yet
- ch09 The Behavior of Solution PDFDocument90 pagesch09 The Behavior of Solution PDFengineerNo ratings yet
- Yo Yo Yo Yo Yo YoDocument3 pagesYo Yo Yo Yo Yo Yoappugt999No ratings yet
- Reaction Areas in AodDocument4 pagesReaction Areas in AodPrakash MishraNo ratings yet
- Chemical Equilibrium IPEDocument6 pagesChemical Equilibrium IPEAdiChemAdi100% (2)
- Melt Reactions: 1.1 Gaseous Interactions With The MeltDocument16 pagesMelt Reactions: 1.1 Gaseous Interactions With The MeltAurlin Cuesta SernaNo ratings yet
- International Journal of Heat and Mass Transfer: Shuangchen Ma, Bin Zang, Huihui Song, Gongda Chen, Jiehong YangDocument8 pagesInternational Journal of Heat and Mass Transfer: Shuangchen Ma, Bin Zang, Huihui Song, Gongda Chen, Jiehong YangusamaNo ratings yet
- Module 13 TransDocument16 pagesModule 13 Transfwong84819No ratings yet
- Hickman 1993Document4 pagesHickman 1993tieNo ratings yet
- Gas Liquid Reactor in The Synthesis of UDocument6 pagesGas Liquid Reactor in The Synthesis of Usaramartori.2002No ratings yet
- Ext. Met 3Document7 pagesExt. Met 3Anubhav ChandilNo ratings yet
- Catalysts 07 00032Document25 pagesCatalysts 07 00032María Martha BQNo ratings yet
- VD Vs RHDocument25 pagesVD Vs RHsathyadevi konnurNo ratings yet
- A2 Chemistry Reaction Kinetics Notes by Sir Muneeb Saleem +923219973304Document19 pagesA2 Chemistry Reaction Kinetics Notes by Sir Muneeb Saleem +923219973304Versha VasdaniNo ratings yet
- Phase Diagrams Distillation Solvent Extraction PDFDocument23 pagesPhase Diagrams Distillation Solvent Extraction PDFchemsac2No ratings yet
- Effect of Impurities On Ultra-Pure Hydrogen Production by Pressure Vacuum Swing AdsorptionDocument37 pagesEffect of Impurities On Ultra-Pure Hydrogen Production by Pressure Vacuum Swing AdsorptionEvminidaNo ratings yet
- 1 PBDocument6 pages1 PBDeva AfrgNo ratings yet
- Catalysts 10 01095Document7 pagesCatalysts 10 01095Frank Joel Herrera ApaesteguiNo ratings yet
- Module 1 Chem Kinetics CatalysisDocument6 pagesModule 1 Chem Kinetics CatalysisAnima MemesNo ratings yet
- Design of Synthetic Egr and Simulation Study of The Effect of Simplified Formulations On The Ignition Delay of Isooctane and N-HeptaneDocument1 pageDesign of Synthetic Egr and Simulation Study of The Effect of Simplified Formulations On The Ignition Delay of Isooctane and N-HeptaneDarío López PintorNo ratings yet
- Actividad 3 - Compressed PDFDocument7 pagesActividad 3 - Compressed PDFLUIS LIBROSNo ratings yet
- AmmoniaDocument2 pagesAmmoniaMuhamad KhairuddinNo ratings yet
- Eudiometry: Nurture CourseDocument16 pagesEudiometry: Nurture CourseAshik jhaNo ratings yet
- Shimadzu - Comparasion of Separation Performance With Various Carrier GasDocument2 pagesShimadzu - Comparasion of Separation Performance With Various Carrier Gasandi wibowoNo ratings yet
- Graham's Gas LawDocument15 pagesGraham's Gas LawAllenNo ratings yet
- 2 Technical Note 11 - 13Document3 pages2 Technical Note 11 - 13Dr. Satish TailorNo ratings yet
- 2.nanoenergy Heterogeneous CatalysisDocument34 pages2.nanoenergy Heterogeneous CatalysisRizki FebrianNo ratings yet
- Solution: SolutionsDocument10 pagesSolution: SolutionsNiranjan RajaNo ratings yet
- Steady State Analysis of A Falling Film PDFDocument10 pagesSteady State Analysis of A Falling Film PDFtreyzzztylerNo ratings yet
- 6A Chemical Energetics IDocument40 pages6A Chemical Energetics IArvin LiangdyNo ratings yet
- International Journal of Greenhouse Gas Control: A. Hartono, S.J. Vevelstad, A. Ciftja, H.K. KnuutilaDocument11 pagesInternational Journal of Greenhouse Gas Control: A. Hartono, S.J. Vevelstad, A. Ciftja, H.K. KnuutilamppatilmayurNo ratings yet
- Handwritten Notes - Chemical Reactions and Equations - Chemical HandwrittenDocument16 pagesHandwritten Notes - Chemical Reactions and Equations - Chemical HandwrittenisharNo ratings yet
- Augusto Moreira 2020Document26 pagesAugusto Moreira 2020Zouhir ZEROUALNo ratings yet
- 2 A Review of Basic Laws - DocDocument17 pages2 A Review of Basic Laws - DocTrường Nguyễn HuyNo ratings yet
- Production of Synthetic Gas From The Gasification of Biomass (Rice Husk)Document1 pageProduction of Synthetic Gas From The Gasification of Biomass (Rice Husk)Bilal AhmadNo ratings yet
- SENTA ITS 2018 - Rizqiana Yogi CahyaningtyasDocument5 pagesSENTA ITS 2018 - Rizqiana Yogi CahyaningtyasRizqiana Yogi CahyaningtyasNo ratings yet
- Enthalpy Change WorksheetDocument3 pagesEnthalpy Change WorksheetChemist Mohamed MohyNo ratings yet
- Modelling of Unsaturated Gas Flow by Thebes Code: Validation TestsDocument6 pagesModelling of Unsaturated Gas Flow by Thebes Code: Validation TestsAyman ABEDNo ratings yet
- Evans2016 3Document36 pagesEvans2016 3EnriqueNo ratings yet
- Homogeneous and Heterogeneous CombustionDocument18 pagesHomogeneous and Heterogeneous CombustionGiova RossiNo ratings yet
- Lecture 4Document3 pagesLecture 4espinosajennywayneNo ratings yet
- Chapter 4 BDocument5 pagesChapter 4 BAndy LêNo ratings yet
- Gases: Ideal Gases (Ideal Gas Law)Document2 pagesGases: Ideal Gases (Ideal Gas Law)Akib ImtihanNo ratings yet
- Haber ProcessDocument2 pagesHaber ProcessDroid4x BevinNo ratings yet
- Inorganic Chemistry: Learning Module inDocument8 pagesInorganic Chemistry: Learning Module inKevinNo ratings yet
- Engineering Degree in Oil Management Properties of Natural Gas Alexander Marcel Zeballos Panozo Ninth Semester Engineering Degree in Oil ManagementDocument5 pagesEngineering Degree in Oil Management Properties of Natural Gas Alexander Marcel Zeballos Panozo Ninth Semester Engineering Degree in Oil Managementvictor nuñezNo ratings yet
- Properties and Behavior of AirDocument6 pagesProperties and Behavior of AirsifatNo ratings yet
- 1 Silaen Wang IPCC2009Document11 pages1 Silaen Wang IPCC2009mohammadjm2008No ratings yet
- The Using of Alkanes in The Role of FuelDocument1 pageThe Using of Alkanes in The Role of FuelCosmescu Mario FlorinNo ratings yet
- CH 8. Chemical Equilibrium (Chem +1)Document40 pagesCH 8. Chemical Equilibrium (Chem +1)Dipin Preet SinghNo ratings yet
- Atmosphere: A Theoretical Study of The N + H Reactive Collisions For High Vibrational and Translational EnergiesDocument16 pagesAtmosphere: A Theoretical Study of The N + H Reactive Collisions For High Vibrational and Translational EnergiesMonica Ionita-ScholzNo ratings yet
- Gasifier W: SyngasDocument8 pagesGasifier W: SyngascristopuloNo ratings yet
- UREA Presentation - PPTMDocument33 pagesUREA Presentation - PPTMRao QamarNo ratings yet
- 10-Part DocumentaryDocument1 page10-Part DocumentaryPeer MohamedNo ratings yet
- Odd Week Timetable: NPCC Training NPCC TrainingDocument1 pageOdd Week Timetable: NPCC Training NPCC TrainingPeer MohamedNo ratings yet
- 2023 Sec 4/5 WA2 Schedule: Good FridayDocument1 page2023 Sec 4/5 WA2 Schedule: Good FridayPeer MohamedNo ratings yet
- Tone/Attitude (List)Document2 pagesTone/Attitude (List)Peer MohamedNo ratings yet
- Design and TechnologyDocument1 pageDesign and TechnologyPeer MohamedNo ratings yet
- Social Media Annotations EnglishDocument3 pagesSocial Media Annotations EnglishPeer MohamedNo ratings yet
- Geography Social Impacts Mind MapDocument1 pageGeography Social Impacts Mind MapPeer MohamedNo ratings yet
- Installation Instructions For Trimless in Drywall: Trimless CabinetsDocument2 pagesInstallation Instructions For Trimless in Drywall: Trimless Cabinetsdonaji123No ratings yet
- Group Case Study: Course: MGT314 Section: 6 Group: 5Document5 pagesGroup Case Study: Course: MGT314 Section: 6 Group: 5Nextdoor CosplayerNo ratings yet
- Dow Corning - Fabricating With XIAMETER® High Consistency Silicone Rubber PDFDocument50 pagesDow Corning - Fabricating With XIAMETER® High Consistency Silicone Rubber PDFLin NiuNo ratings yet
- Report LatheDocument4 pagesReport LatheMike Pioquinto0% (1)
- Rip ProtectionDocument4 pagesRip ProtectionnicolasNo ratings yet
- Section 1 Abrasive CDIDocument14 pagesSection 1 Abrasive CDINurul RiskiyaNo ratings yet
- MS 5357 - B Norma ZincDocument6 pagesMS 5357 - B Norma Zincfernando.zabdiNo ratings yet
- Kundalia RB Project - Cage Ladder QAPDocument2 pagesKundalia RB Project - Cage Ladder QAPHarshit AdwaniNo ratings yet
- Stainless Steel Grade 202 (UNS S20200)Document3 pagesStainless Steel Grade 202 (UNS S20200)MuhammadJawadNo ratings yet
- 3236 P&ID SummaryDocument5 pages3236 P&ID Summarysource codeNo ratings yet
- Specification For Aluminum-Alloy Sand Castings: SB-26 /SB-26MDocument17 pagesSpecification For Aluminum-Alloy Sand Castings: SB-26 /SB-26MHernan RiveraNo ratings yet
- Cemfair SmoothDocument2 pagesCemfair Smoothosama mohNo ratings yet
- Avesta 2205 VDX Ac DCDocument1 pageAvesta 2205 VDX Ac DCpipedown456No ratings yet
- MillingDocument17 pagesMillingarslanNo ratings yet
- Carbo-Austempering - A New WrinkleDocument8 pagesCarbo-Austempering - A New WrinkleMustafa OğuzhanNo ratings yet
- Woodwork: Scheme of ExaminationDocument10 pagesWoodwork: Scheme of ExaminationKunbi Santos-ArinzeNo ratings yet
- Ga Sheracast (Englisch)Document3 pagesGa Sheracast (Englisch)valeriu vatamanNo ratings yet
- TDS Byk-1780 enDocument2 pagesTDS Byk-1780 enabhijit.home2022No ratings yet
- WhiteDocument3 pagesWhiteMERVENo ratings yet
- 146-Article Text-230-1-10-20220511Document13 pages146-Article Text-230-1-10-20220511mintaito tjgNo ratings yet
- 3200 Version 7thDocument2 pages3200 Version 7thDuc NguyenNo ratings yet
- Ceramic Note 11.12.2023Document4 pagesCeramic Note 11.12.2023Nguyen Ha PhuongNo ratings yet
- Experimental Analysis of Rotary Friction Joining Process by EN1A Bright Mild Steel Under Normal Air and Wet Environment ConditionDocument6 pagesExperimental Analysis of Rotary Friction Joining Process by EN1A Bright Mild Steel Under Normal Air and Wet Environment ConditionRafael PriambadhaNo ratings yet
- SIMONA901 ECTFE 901 Data SheetDocument2 pagesSIMONA901 ECTFE 901 Data Sheetdavid leeNo ratings yet
- Me688 UsmDocument39 pagesMe688 UsmKETU PRINCE LEKUNo ratings yet
- Instruction Writing Magnetic Testing For Single Vee Butt WeldDocument5 pagesInstruction Writing Magnetic Testing For Single Vee Butt WeldVu PhamNo ratings yet
- Marathon XHB - English (Uk) - Issued.06.12.2007Document3 pagesMarathon XHB - English (Uk) - Issued.06.12.2007Mohamed FaragNo ratings yet