Professional Documents
Culture Documents
19 2중간
Uploaded by
민규강Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
19 2중간
Uploaded by
민규강Copyright:
Available Formats
Mid-Term Exam (Organic Chemistry, AMIE261-00, Fall 2019)
October 21, 2019, 03:00 pm – 04:15 pm
Name: Prof. Jae Woong Jung
ID number:
Select True (T) or False (F) for each statement. (5 points each)
• For the structure of ethyelene, H atoms form σ bonds with four sp3 orbitals. ( F )
• The organic acids may lose a proton from C–H from a carbon atom next to a C=O double bond. ( F )
• Isomers that differ in how their atoms are arranged in chains are called constitutional isomers. ( T )
• Cycloalkanes are more flexible than open-chain alkanes. ( F )
• Chair conformations readily interconvert, resulting in the exchange of axial and equatorial positions by a ring-flip,
and thus two conformations of monosubstituted cyclohexane are equally stable. ( F )
• Aryl halides and vinylic halides do not react in Fridel-Crafts alkylation reaction because their carbocations are
very unstable to form. ( T )
• For the meta-directors in electrophilic aromatic substitution, Ortho and para intermediates destabilized because
inductive and resonance effects reinforce each other. ( T )
• Resonance forms differ only in the placement of their π or nonbonding electrons. ( T )
1. Give IUPAC names for the following molecules (5 points each)
(a) (b) (c) (d)
2. Predict the major organic product(s) in the reaction below. Suggest the exact reason for each product(s). (5 points each)
(a) (b) (c)
Br2, FeBr3 HBr
3. Determine the configuration of the carbon atom labeled with an arrow for following molecules. (5 points each)
(a) (b)
4. Determine the pair of molecules below as enantiomers, diastereomers, or identical. (5 points each)
(a) (b)
5. Draw the major product of the following reaction. (5 points)
You might also like
- 2 CH241 Polar Covalent BondsDocument94 pages2 CH241 Polar Covalent Bondsalyssa_marie_keNo ratings yet
- For The Following Cyclic Compounds, Determine Whether Each Is Aromatic, Anti-Aromatic or Non-AromaticDocument11 pagesFor The Following Cyclic Compounds, Determine Whether Each Is Aromatic, Anti-Aromatic or Non-AromaticRaja AkmalNo ratings yet
- CHEM1280 2011 Midterm Exam PDFDocument3 pagesCHEM1280 2011 Midterm Exam PDFLouisNo ratings yet
- U2 AOL Unit TEstDocument4 pagesU2 AOL Unit TEstanjana ghelaniNo ratings yet
- CH 231 Old Exams 1 and 2Document13 pagesCH 231 Old Exams 1 and 2Brian DaSilva100% (3)
- Exam II - '05Document3 pagesExam II - '05kitthiNo ratings yet
- SEM 5 - Paper VI 2021 MarchDocument6 pagesSEM 5 - Paper VI 2021 MarchsankarNo ratings yet
- CHEM231 - Assignment 2 - 2021-KeyDocument12 pagesCHEM231 - Assignment 2 - 2021-Keyzoya attiqueNo ratings yet
- Tutorial Letter 201/2/2017: General Chemistry 1BDocument16 pagesTutorial Letter 201/2/2017: General Chemistry 1BLeigh MakanNo ratings yet
- Class X BAT 1 - CHEM 2ND 50% SLIP TEST-IIDocument2 pagesClass X BAT 1 - CHEM 2ND 50% SLIP TEST-IIphysicsbooks.storeNo ratings yet
- T6c - Alkenes BookletDocument11 pagesT6c - Alkenes BookletRayhan MessousNo ratings yet
- Post Mid Term9th PaperDocument7 pagesPost Mid Term9th PaperJyoti SumanNo ratings yet
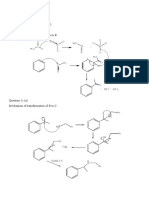
- Question 5C (I) Reagents - Lewis Acid (Alcl3) - Acetyl Chloride (CH Cocl) Mechanism of Transformation of A To BDocument9 pagesQuestion 5C (I) Reagents - Lewis Acid (Alcl3) - Acetyl Chloride (CH Cocl) Mechanism of Transformation of A To BKIBET SHADRACKNo ratings yet
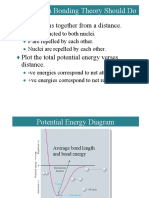
- 11-1 What A Bonding Theory Should Do: Bring Atoms Together From A DistanceDocument85 pages11-1 What A Bonding Theory Should Do: Bring Atoms Together From A DistanceNazım Bora YanıkNo ratings yet
- 2014 DecDocument5 pages2014 DecBuyuNo ratings yet
- Science Class 10 Term 2 Sample Paper 2Document5 pagesScience Class 10 Term 2 Sample Paper 2Sangeeta SharmaNo ratings yet
- OC Part B QuestionsDocument10 pagesOC Part B QuestionsSunanda 2004No ratings yet
- Chemistry Related Exam Questions and AnswersDocument11 pagesChemistry Related Exam Questions and AnswersJoseph NyabugaNo ratings yet
- (Ser-2) SR Neet Star Super Chaina (Neet Grand Test-4) Q.P Ex - Dt. 22.04.2024Document21 pages(Ser-2) SR Neet Star Super Chaina (Neet Grand Test-4) Q.P Ex - Dt. 22.04.2024lavendar31selenNo ratings yet
- Aromatic Chemistry PDFDocument10 pagesAromatic Chemistry PDFIsmaelovfNo ratings yet
- Null 5Document6 pagesNull 5gamerzsilent69No ratings yet
- Aromatic ChemistryDocument10 pagesAromatic Chemistryilias1973No ratings yet
- Answers 7Document22 pagesAnswers 7SureshkumaryadavNo ratings yet
- Chemistry 1Document2 pagesChemistry 1chikondikosamu24No ratings yet
- 02 - CB Tut (B1-5 Ans)Document5 pages02 - CB Tut (B1-5 Ans)2022 BALAKRISHNAN ADHITHINo ratings yet
- AP Bonding Questions Answer KeyDocument4 pagesAP Bonding Questions Answer KeyMysticNo ratings yet
- Chemistry 3719 Old ExamsDocument300 pagesChemistry 3719 Old ExamsHY-11 Đỗ Quốc TiệpNo ratings yet
- 10523-Etarjome EnglishDocument7 pages10523-Etarjome Englishtieum9761No ratings yet
- Isomerism and Stereochemistry: Answers To Worked ExamplesDocument15 pagesIsomerism and Stereochemistry: Answers To Worked ExamplesDana CapbunNo ratings yet
- 407 ChemistryDocument2 pages407 ChemistrybholuNo ratings yet
- CHEM 33 Extra Practice 05 AnswersDocument7 pagesCHEM 33 Extra Practice 05 Answershuang.sundi3134No ratings yet
- Chemical Bonding NotesDocument4 pagesChemical Bonding NotesvisheshNo ratings yet
- Organic - Reagents FinalDocument27 pagesOrganic - Reagents FinalSankar AdhikariNo ratings yet
- Candidates Are Required To Give Their Answers in Their Own Words As Far As Practicable. The Figures in The Margin Indicate Full MarksDocument2 pagesCandidates Are Required To Give Their Answers in Their Own Words As Far As Practicable. The Figures in The Margin Indicate Full MarkssushilNo ratings yet
- 303 - 11 Final Exam KEY-1 PDFDocument22 pages303 - 11 Final Exam KEY-1 PDFaegaisNo ratings yet
- BotanyDocument24 pagesBotanyMehnaz NazirNo ratings yet
- Problem Set 3Document3 pagesProblem Set 3Aya HachanaNo ratings yet
- Isomerism One Shot BouncebackDocument196 pagesIsomerism One Shot BouncebackHarishNo ratings yet
- Anic Chemistry AK 2018-19Document22 pagesAnic Chemistry AK 2018-19XXXNo ratings yet
- 05 Valence Shell Electron Pair RepulsionDocument34 pages05 Valence Shell Electron Pair RepulsionTitobiloluwa AlbertNo ratings yet
- Focus Area Unit 4Document5 pagesFocus Area Unit 4Muhammed Muhasin. KNo ratings yet
- 4.1 Lewis Structure Lecture With IJJDocument69 pages4.1 Lewis Structure Lecture With IJJIvy JNo ratings yet
- Practice Questions - Weeks 345 - 3rdedDocument5 pagesPractice Questions - Weeks 345 - 3rdedJon LevinsNo ratings yet
- CHEM Test 2 SolutionsDocument5 pagesCHEM Test 2 Solutionscuongtran_siegenNo ratings yet
- BCHCT-131 em 2023@7736848424Document23 pagesBCHCT-131 em 2023@7736848424Anshit GuptaNo ratings yet
- Class XII Chemistry (Code - 043) Sample Question Paper 2018-19 Time Allowed: 3 Hours Max. Marks: 70Document7 pagesClass XII Chemistry (Code - 043) Sample Question Paper 2018-19 Time Allowed: 3 Hours Max. Marks: 70Devansh ShrivastavaNo ratings yet
- Cellona PS1Document5 pagesCellona PS1Andrew John CellonaNo ratings yet
- CHM361 - CHAPTER 1 Valence Bond Theory 2Document57 pagesCHM361 - CHAPTER 1 Valence Bond Theory 2EhazNo ratings yet
- 4.06 4.07 HL Chemical Bonding StructureDocument79 pages4.06 4.07 HL Chemical Bonding StructureSai MedhanshNo ratings yet
- CML 100 QuestionsDocument7 pagesCML 100 QuestionsamanNo ratings yet
- CML 100 Questions PDFDocument7 pagesCML 100 Questions PDFDivyansh GuptaNo ratings yet
- Molecular GeometryDocument85 pagesMolecular GeometryKeri Gobin SamarooNo ratings yet
- MotDocument21 pagesMotDheeraj PradeepNo ratings yet
- Lecture 35 PDFDocument20 pagesLecture 35 PDFRachit ShahNo ratings yet
- Instructions: Part A Part B.: Separate Answer Scripts Are To Be Used For Part A and Part BDocument3 pagesInstructions: Part A Part B.: Separate Answer Scripts Are To Be Used For Part A and Part BAnurag TiwariNo ratings yet
- 2562726-Class 12 - Unit Test - Chemistry - Set 1 - Jenifer - QPDocument4 pages2562726-Class 12 - Unit Test - Chemistry - Set 1 - Jenifer - QPkjfnk,jgNo ratings yet
- Glance Through The Entire Questions and Solve The Easiest Problems FirstDocument4 pagesGlance Through The Entire Questions and Solve The Easiest Problems FirstRutul JainNo ratings yet
- Answers To Chapter 1 In-Chapter ProblemsDocument13 pagesAnswers To Chapter 1 In-Chapter ProblemsChinmay DabkeNo ratings yet
- Metallabenzenes: An Expert ViewFrom EverandMetallabenzenes: An Expert ViewL. James WrightNo ratings yet
- Chapter 6Document34 pagesChapter 6민규강No ratings yet
- Chapter 7Document37 pagesChapter 7민규강No ratings yet
- Chapter 13Document27 pagesChapter 13민규강No ratings yet
- Chapter 8Document41 pagesChapter 8민규강No ratings yet
- Chapter 4Document43 pagesChapter 4민규강No ratings yet
- Chapter 1Document48 pagesChapter 1민규강No ratings yet
- Chapter 3Document38 pagesChapter 3민규강No ratings yet
- Appendix2 ThiladomideDocument8 pagesAppendix2 Thiladomide민규강No ratings yet
- Chapter 2Document48 pagesChapter 2민규강No ratings yet
- Appendix1 MorphineDocument75 pagesAppendix1 Morphine민규강No ratings yet