Professional Documents
Culture Documents
Chemistry Reviewer
Uploaded by
Jayrille EstarezOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemistry Reviewer
Uploaded by
Jayrille EstarezCopyright:
Available Formats
Chemistry Reviewer
Solubility Product Constants, Ksp
Solubility product constants are used to describe saturated solutions of ionic compounds of relatively low
solubility.
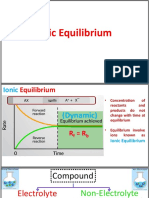
A saturated solution is in a state of dynamic equilibrium between the dissolved, dissociated, ionic
compound and the undissolved solid.
The solubility product constant Ksp, is the equilibrium constant for a solid substance dissolving in an aqueous
solution.
This is shown below:
Calculating the Solubility of an Ionic Compound in Pure Water from its Ksp
Example: Estimate the solubility of Ag2CrO4 in pure water if the solubility product constant for silver chromate
is 1.1 x 10-12.
Write the equation and the equilibrium expression.
Ag2CrO4(s) --> 2 Ag+(aq) + CrO42-(aq)
Ksp = [Ag+]2[CrO42-]
Make an "ICE" chart.
Let "x" be the number of moles of silver chromate that dissolves in every liter of solution (its solubility).
CrO42-(aq) CrO42-(aq)
Ag2CrO4(s)
Initial Concentration 0 0
Change in Concentration +2x +x
Equilibrium Concentration 2x x
Substitute the equilibrium amounts and the Ksp into the equilibrium expression and solve for x.
1.1 x 10-12 = [2x]2[x]
x = 6.50 x 10-5 M
Galvanic Cell
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively,
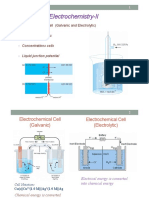
is an electrochemical cell in which an electric current is generated from spontaneous redox reactions.
An electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical
energy is known as a galvanic cell or a voltaic cell.
An electrochemical cell that makes use of chemical reactions to generate electrical energy.
Parts of Galvanic Cell
Anode – Oxidation occurs at this electrode.
Cathode – Reduction occurs at this electrode.
Salt bridge – Contains electrolytes which are required to complete the circuit in a galvanic cell.
Half-cells – reduction and oxidation reactions are separated into compartments.
External circuit – Conducts the flow of electrons between electrodes
Load – A part of the circuit utilizes the electron to flow to perform its function.
Leclanché cell/ dry cell
The Leclanché cell is a battery invented and patented by the French scientist Georges Leclanché in 1866.
A dry cell is a type of electric battery, commonly used for portable electrical devices. It was developed in 1886
by the German scientist Carl Gassner, after development of wet zinc-carbon batteries by Georges Leclanché in
1866.
The modern version was developed by Japanese Yai Sakizo in 1887.
A standard dry cell comprises a zinc anode, usually in the form of a cylindrical pot, with a carbon cathode
in the form of a central rod. The electrolyte is ammonium chloride in the form of a paste next to the zinc
anode.
Uses of Dry Cell
Electronic Equipment
Dry cell batteries appear as the most commonly used battery type for powering electronic devices.
You might also like
- CH 3 14Document135 pagesCH 3 14active learning educationNo ratings yet
- Summary Sa CHEMISTRYDocument12 pagesSummary Sa CHEMISTRYHazel BayanoNo ratings yet
- Shambhugowda Lecturer in ChemistryDocument30 pagesShambhugowda Lecturer in ChemistryAryan Sai ANo ratings yet
- Chapter 18 ElectrochemistryDocument17 pagesChapter 18 ElectrochemistryNefliNo ratings yet
- Electrochemistry 2019 HANDOUTDocument50 pagesElectrochemistry 2019 HANDOUTAndrearose Ivy FietasNo ratings yet
- ELECTROCHEMISTRYDocument33 pagesELECTROCHEMISTRYSanthosh AnanthNo ratings yet
- ELECTROCHEMISTRYDocument176 pagesELECTROCHEMISTRYgsvssumaNo ratings yet
- CHEM (Gyana)Document5 pagesCHEM (Gyana)MCHNo ratings yet
- 1.ElectrochemistryPROBLEM SOLVING TACTICSFormulae SheetDocument4 pages1.ElectrochemistryPROBLEM SOLVING TACTICSFormulae SheetArbab MazharNo ratings yet
- Lecture 4 - Electro Chem PDFDocument49 pagesLecture 4 - Electro Chem PDFHedric VillenaNo ratings yet
- Chapter 18 - Rev PDFDocument17 pagesChapter 18 - Rev PDFalaa al sahmaraniNo ratings yet
- ELECTROCHEMISTRYDocument22 pagesELECTROCHEMISTRYmanishkushwah640No ratings yet
- Electrolysis in Aqueous SolutionDocument15 pagesElectrolysis in Aqueous SolutionEdon BediNo ratings yet
- Chapter 18 ElectrochemistryDocument49 pagesChapter 18 ElectrochemistryDwivelia AftikaNo ratings yet
- Chemi G 10 Chap 3 HanoutDocument5 pagesChemi G 10 Chap 3 HanoutYISHAK ABRHAMNo ratings yet
- Practical 1 ElectrolysisDocument27 pagesPractical 1 ElectrolysisGeorge chaupi NyondoNo ratings yet
- Physical Chemistry Chapter 4 - ElectrochemistryDocument36 pagesPhysical Chemistry Chapter 4 - Electrochemistryjatropos6810No ratings yet
- CHE 323 Electrochemisty Problem Set 3-16-17Document2 pagesCHE 323 Electrochemisty Problem Set 3-16-17Zhu Chen ChuanNo ratings yet
- Redox Reaction and Electrochemistry 2018Document66 pagesRedox Reaction and Electrochemistry 2018Jonathan AndikaNo ratings yet
- v0.3 Electrolysis ID1324Document54 pagesv0.3 Electrolysis ID1324tamimihsan2763No ratings yet
- Electrochemistry: Applications of RedoxDocument40 pagesElectrochemistry: Applications of Redoxcatsathish1No ratings yet
- ElectrochemistryDocument49 pagesElectrochemistrySafril JuntakNo ratings yet
- Electrochemistry PPT NotesDocument23 pagesElectrochemistry PPT NotesHemanshi KocharNo ratings yet
- Electrochemistry - Galvanic Cell NewDocument51 pagesElectrochemistry - Galvanic Cell NewPink WandererNo ratings yet
- Che 323 Problem Set 6: Electrochemistry March 12, 2014 Engr. May V. Tampus ConceptsDocument1 pageChe 323 Problem Set 6: Electrochemistry March 12, 2014 Engr. May V. Tampus ConceptsLouie G NavaltaNo ratings yet
- Solubility Product Constants, KDocument4 pagesSolubility Product Constants, KAngeline SmithNo ratings yet
- Electrochemistry Chemical Change and Electrical WorkDocument32 pagesElectrochemistry Chemical Change and Electrical Workmakondo.yhNo ratings yet
- Electrochem Practice TestDocument41 pagesElectrochem Practice TestaaaaaNo ratings yet
- Module 4Document50 pagesModule 4Abi VANo ratings yet
- Electrochemistry Electrochemistry: GCC Chm152Document9 pagesElectrochemistry Electrochemistry: GCC Chm152Star LightNo ratings yet
- Electrochemistry: Compiled By: E.S. EspirituDocument123 pagesElectrochemistry: Compiled By: E.S. EspirituCyrus Vizon100% (1)
- Electrochemical Cells R - Virtual LabDocument3 pagesElectrochemical Cells R - Virtual LabJosua VivasNo ratings yet
- Faculty of Applied Sciences Electrochemistry Chm578 Laboratory Report Experiment 1: Galvanic CellDocument14 pagesFaculty of Applied Sciences Electrochemistry Chm578 Laboratory Report Experiment 1: Galvanic CellimizzNo ratings yet
- CH 20 Electrochemistry:: Review Redox ReactionsDocument27 pagesCH 20 Electrochemistry:: Review Redox ReactionsAtlas shabuNo ratings yet
- Ionic EquilibriumDocument14 pagesIonic Equilibrium8842 AnuragNo ratings yet
- Potentiometry 2023 - PL - BPDocument58 pagesPotentiometry 2023 - PL - BPfojirof555No ratings yet
- CH ElectreochemistryDocument43 pagesCH ElectreochemistryOP HBSNo ratings yet
- Chapter Five Introduction To Electroanalytical ChemistryDocument16 pagesChapter Five Introduction To Electroanalytical ChemistryZekarias LibenaNo ratings yet
- Lecture 1 ElektrokimiaDocument58 pagesLecture 1 ElektrokimiaVARUNESH A/L MAUTHIALAGANNo ratings yet
- Kimia Teknik 6Document52 pagesKimia Teknik 6Febry MiftakhulNo ratings yet
- 5 ElectrochemistryDocument60 pages5 Electrochemistrynirvanjain212007No ratings yet
- +2 Chem Ultimate Question BankDocument253 pages+2 Chem Ultimate Question Bankflex93948No ratings yet
- 10 Chapter Electrochemistry Short Question With Answers PDFDocument11 pages10 Chapter Electrochemistry Short Question With Answers PDFMARITIM GEOFFREY KIPLANGATNo ratings yet
- Electrochemical Energy Systems DV 2021 EvenDocument116 pagesElectrochemical Energy Systems DV 2021 EvenAditi BardhanNo ratings yet
- Balance The Following Oxidation-2Document9 pagesBalance The Following Oxidation-2Justin FolkNo ratings yet
- Chapter 6-ElectrolysisDocument78 pagesChapter 6-Electrolysismubashira.5031No ratings yet
- Electrochemistry Chemistry and ElectricityDocument54 pagesElectrochemistry Chemistry and ElectricityMaria OzaoNo ratings yet
- 3 ElectrochemDocument4 pages3 ElectrochemFelven Leo AbayaNo ratings yet
- B. Electrochemistry 2 1Document37 pagesB. Electrochemistry 2 1Dank CoderNo ratings yet
- ElectrochemistryDocument63 pagesElectrochemistryomer faruqeNo ratings yet
- Voltaic and Electrolytic Cell ComparisonDocument32 pagesVoltaic and Electrolytic Cell Comparisonmamta2111No ratings yet
- Chapter 4 Chm271Document63 pagesChapter 4 Chm271Amirah AdlinaNo ratings yet
- Chapter 18 ElectrochemistryDocument53 pagesChapter 18 ElectrochemistryKatarina WuriyaniNo ratings yet
- Determination of Dissociation Constant From Conductivity MeasurementsDocument7 pagesDetermination of Dissociation Constant From Conductivity MeasurementsRaluca IosuNo ratings yet
- ElectrochemistryDocument10 pagesElectrochemistrySsNo ratings yet
- Electrochemistry Part 2Document23 pagesElectrochemistry Part 2ABHINAVNo ratings yet
- CH-110, Lecture 1Document29 pagesCH-110, Lecture 1Naveed TanoliNo ratings yet
- NEET UG Chemistry Redox Reactions and ElectrochemistryDocument24 pagesNEET UG Chemistry Redox Reactions and ElectrochemistryAmanNo ratings yet
- Critical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsFrom EverandCritical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsNo ratings yet
- Composting 2019 20Document34 pagesComposting 2019 20BhavaNo ratings yet
- Technical Reference On Hydrogen Compatibility of Materials: Austenitic Stainless Steels: Type 304 & 304L (Code 2101)Document19 pagesTechnical Reference On Hydrogen Compatibility of Materials: Austenitic Stainless Steels: Type 304 & 304L (Code 2101)nktiah1207No ratings yet
- Ministry of Railways Manak Nagar, Lucknow 226 011Document24 pagesMinistry of Railways Manak Nagar, Lucknow 226 011Sameer KattiNo ratings yet
- Experiment No. 3 - PermanganometryDocument3 pagesExperiment No. 3 - PermanganometryKatherine A. PerezNo ratings yet
- Plumbing CodeDocument131 pagesPlumbing CodeJysar Reubal0% (1)
- Brochure Dramix 5D4D3DDocument32 pagesBrochure Dramix 5D4D3Dpsychoo4702No ratings yet
- SPE 25892 Field Implementation of Proppant Slugs To Avoid Premature Screen-Out of Hydraulic Fractures With Adequate Proppant ConcentrationDocument16 pagesSPE 25892 Field Implementation of Proppant Slugs To Avoid Premature Screen-Out of Hydraulic Fractures With Adequate Proppant ConcentrationbayuNo ratings yet
- Eals Q1 SLMDocument24 pagesEals Q1 SLMJanice DagamacNo ratings yet
- Genesol 37: Strongly Acidic Membrane CleanerDocument1 pageGenesol 37: Strongly Acidic Membrane CleanerOsama AhmedNo ratings yet
- Kao Corporation, S.A.: Disinfecting Floor Cleaner Containing Ammoniac & Pine OilDocument2 pagesKao Corporation, S.A.: Disinfecting Floor Cleaner Containing Ammoniac & Pine OilAlna Technical100% (1)
- Velammal Vidyalaya: Section A (Objective Type)Document7 pagesVelammal Vidyalaya: Section A (Objective Type)Ashwath SaiNo ratings yet
- Ipc2010 31293Document10 pagesIpc2010 31293Sriven ReddyNo ratings yet
- Module 51Document11 pagesModule 51Zac IriberriNo ratings yet
- A313 Pharma DeveleppomentDocument5 pagesA313 Pharma Develeppomentdenemegaranti78No ratings yet
- Aquafin-2K/M Two-Component Cement-Based Acrylic-Based Waterproofing Guide Aquafin, Inc. SpecificationDocument5 pagesAquafin-2K/M Two-Component Cement-Based Acrylic-Based Waterproofing Guide Aquafin, Inc. SpecificationalpegambarliNo ratings yet
- Merino Residential Vanity - Catalogue 2023-CompressedDocument22 pagesMerino Residential Vanity - Catalogue 2023-CompressedSudip DuttaNo ratings yet
- Spirajet Spray NozzleDocument2 pagesSpirajet Spray NozzleykozeNo ratings yet
- ESTOP-TDS-Estowrap 300 & 600Document5 pagesESTOP-TDS-Estowrap 300 & 600Dona Dwi SaputroNo ratings yet
- CastingDocument29 pagesCastingKumar HemjeetNo ratings yet
- Welding Journal 1961 7Document159 pagesWelding Journal 1961 7AlexeyNo ratings yet
- Cowon BT 401Document3 pagesCowon BT 401BradNo ratings yet
- Jeori: SEMINAR (CE-613)Document21 pagesJeori: SEMINAR (CE-613)Tarun jajuaNo ratings yet
- Project Report On Fused Salt Solution ElectrolysisDocument9 pagesProject Report On Fused Salt Solution Electrolysisvaibhav12345678No ratings yet
- Solid Waste Management Scenerio in GuwahatiDocument6 pagesSolid Waste Management Scenerio in GuwahatiUsüî Jack Martin TakumiNo ratings yet
- Capítulo 6Document83 pagesCapítulo 6fslsantosNo ratings yet
- Fortune Laser FL-S6020Document11 pagesFortune Laser FL-S6020wayzxc775No ratings yet
- Fathi Habashi PYRITE THE STRATEGIC MINERAL THAT BECAME AN INDUSTRIAL NUISANCE PDFDocument16 pagesFathi Habashi PYRITE THE STRATEGIC MINERAL THAT BECAME AN INDUSTRIAL NUISANCE PDFboanerges wino pattyNo ratings yet
- Fire Resistance Assessment of Concrete StructuresDocument81 pagesFire Resistance Assessment of Concrete StructuresJevgenijsKolupajevsNo ratings yet
- F1 C7englishDocument66 pagesF1 C7englishMexlyn AdnanNo ratings yet