Professional Documents
Culture Documents
Unit 8 Acids and Bases One Pager
Uploaded by
asmaa eOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Unit 8 Acids and Bases One Pager
Uploaded by
asmaa eCopyright:
Available Formats
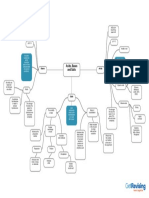
Unit 8 Acids and Bases
1 1
d Titration Curves
Htt CHzCHzCÉ fÉ
agency
H
j a
concentration 841
strongacid Ht andstrongBase
14
Fettesson If dÉÉ
calculatednot
weak acids partially ionize in
water to produce Hzotions
P t tnotdan
cotDwater
47mi
water is amphoteric can behave as an acid or base
ReactionQuotient
Quantitiesof products or
rxnoIctD
caIII.IE it
calcwith
water Ka of weak acid
Acid dissociation
ii constant A
og meon equivalence p measure of acid strength
a
tf a
coz thecost of m.mn
solids is and purecounsel weakacidhaltstrong Base excesson is En't to
ka I for a weakacid
A solution ofweakacidinvolves
pts a cantthocietacaaltoticaa
qq.gesscon.fm
are not included in Q because
ptrateavivalencepointisbasic
equilibriumbetween an un ionized
thot cos Hot Qc I Qp IcaIIeauivaenap
of ran baseand
water betweenconjugate
Egg.jo
coz not base
that is astrong because
Base acid K Equilibriumconstant usedinstead of one we
v point and
e'avivalenceptivieantaience
it's conjugate
resistschange in
co
upon
pka 10g ka
Q when a system is at equilibrium addition of on ya
941
Kc o weakbase Y strongacid when Ctia Cat pHpka
ptl logCtizot potts logCott HendersonHasselbach when Cha Ca J propria
pts pratlog Eas
gyppxygggiiiiiiiiiiiiiiii phat equivalence
of ran
not
pointsac
acidwith
betweenconjugate
because'trant d
water
astros
when CHA CaT pHpka
d
migf.ggigiinEIm
o and
contributionis
or pressures of reactantsand excesstitltyte
products Iftemperature changes
BufferzoneweakBasealandit's
basecat
conjugate Largerkalkbs strongeracidbase
Autoionization of water K will change smamp.am onsuaad
tho it thou head min Buffer zones
weak acid strongBase Acid
weakbase strong
BASE ACID CA CB DYE.ci pfo8ffI G'fauokured n
weak basespartiallyionize in
iffy
water to produce oh ions
Kw CHzOtJCotiJ 250C.k 1 10
1 c
gigginied roars
mm Kb of weak base
verysmall F strongest E
ie if reactants arefavoured F as acid strengtht
o asstrengthofbased involvesequilibrium between
µ K is smaller a
startingpts buffer
zone staring put an un ionized baseandit's
Bissersumpt Biggerdropa buffer
zone
K I products reactants conjugate acid
ma on
Inf's'afetrensatestrength't
In eats'trenstat
K Cl products c reactants strongBases
Kb CHB'TCOAT pkb logKb
strongacids
pure water Fully associate in water
to producerotations
ftp.dissogateionnswaterto
ciote.naoti.rote.rboti.com
CB
Chzot
pKw 14.0 ptl
COM pH putt 7 14
log l 0 10 25 C
K reactiongoesto completion
1000
no reverse reaction
na ter tee cos
no andMason 88111sm'etaffydroxides f fÉÉÉÉÉÉÉ
IIas
cazot decreases
ionizationincreases
00
m K co 0001 the forward reactiondoes
me Torgina
As temperature increasesTpkwdecreasest Example Buffers pHrange pka II
As temperature increases i kwincreases
endothermic
At Equilibrium Q K
if Q K the numeratormustdecrease
to make Q K Products decrease
original
Addjoominen
100.0miofo.iomticizoo.omofo.us
mad
In
concentrationhalves
ge n p
1.30 1.00 0.30
a solutionthatresistschangeinpH
when asmallamountofstrongacid
or it'sbaseisaddedtosolution
Temp
and reverse reactionis favoured 200.0mi of0.50MNaOH 0.507102004410.300
no ing or weakbaseand it's c a salt
0.33M x sourced for e
aa G3 10
15 If Qe K the numeratormustincrease e g teno and no g
to make Q k the productsmust 300.0miof o33mn aot13.52 13.70 0.17 fromsodiumsaltof cA likenano
25 in o i ox 300.0midistilledwater 400.0midistilledwater pH7.00
increase and forward Reaction Chzot ka ptl pkawhen
30 13 8 1 oxio The largerthe change in concentration nochange concentrationsofweak
acidand
3 2 10
1 ng am conjugatebase are equal
You might also like
- Acid Mind MapDocument1 pageAcid Mind MapIndianagrofarms100% (2)
- Budget of Work Chem 2 Midterm Curriculum MappiDocument23 pagesBudget of Work Chem 2 Midterm Curriculum MappiIm NaYeon TWICENo ratings yet
- List of Laboratories in KZN and GautengDocument6 pagesList of Laboratories in KZN and GautengLungisani50% (2)
- Water Soluble Vitamin - B5 - B7Document4 pagesWater Soluble Vitamin - B5 - B7Pravat Ranjan BeheraNo ratings yet
- Chapter 1, Unit 2, Pharmaceutical Analysis, B Pharmacy 1st Sem, Carewell PharmaDocument9 pagesChapter 1, Unit 2, Pharmaceutical Analysis, B Pharmacy 1st Sem, Carewell PharmaIndian TechnicalNo ratings yet
- Lat - Long Verification SummaryDocument31 pagesLat - Long Verification SummaryAshish TiwariNo ratings yet
- Adobe Scan 02-Feb-2023Document3 pagesAdobe Scan 02-Feb-2023Lokesh SenNo ratings yet
- Summary of Nephron Action - FactRecallDocument1 pageSummary of Nephron Action - FactRecallsabinaNo ratings yet
- Eposter - 29359 - Acute Necrotizing PancreatitisDocument1 pageEposter - 29359 - Acute Necrotizing PancreatitisVanyieldNo ratings yet
- Unit 1, Pharmaceutical Analysis, B Pharmacy 1st Sem, Carewell PharmaDocument14 pagesUnit 1, Pharmaceutical Analysis, B Pharmacy 1st Sem, Carewell PharmaSafia WahidNo ratings yet
- Chapter 2, Unit 2, Pharmaceutical Analysis, B Pharmacy 1st Sem, Carewell PharmaDocument7 pagesChapter 2, Unit 2, Pharmaceutical Analysis, B Pharmacy 1st Sem, Carewell Pharmayash08jan01No ratings yet
- Ma DPH Covid-19 DashboardDocument17 pagesMa DPH Covid-19 DashboardJohn Waller100% (1)
- Note Aug 23, 2023Document4 pagesNote Aug 23, 2023Meghan IrishNo ratings yet
- Financial Ratio Analysis: Submitted TODocument16 pagesFinancial Ratio Analysis: Submitted TOShahriar Ali DolonNo ratings yet
- CRE Exp4Document3 pagesCRE Exp4kabali007123No ratings yet
- Ma DPH Covid-19 DashboardDocument17 pagesMa DPH Covid-19 DashboardRami Abou-SabeNo ratings yet
- Ma DPH Covid-19 DashboardDocument17 pagesMa DPH Covid-19 DashboardJohn WallerNo ratings yet
- Ma DPH Covid-19 DashboardDocument17 pagesMa DPH Covid-19 DashboardJohn WallerNo ratings yet
- Ma DPH Covid-19 DashboardDocument17 pagesMa DPH Covid-19 DashboardJohn WallerNo ratings yet
- Ma DPH Covid-19 DashboardDocument17 pagesMa DPH Covid-19 DashboardJohn WallerNo ratings yet
- R. NI KI L Kumar - Archi T ECT Ural DES I GN-I V - 921418251011 - RVS S OA - S Heet NO. 1 Conventi ON CentreDocument1 pageR. NI KI L Kumar - Archi T ECT Ural DES I GN-I V - 921418251011 - RVS S OA - S Heet NO. 1 Conventi ON Centrenikil sekaNo ratings yet
- Dashboard 1 PDFDocument1 pageDashboard 1 PDFWade ZhangNo ratings yet
- Adobe Scan Dec 20, 2022Document1 pageAdobe Scan Dec 20, 2022Prathmesh GhodvindeNo ratings yet
- IgneasDocument39 pagesIgneasJose Antonio Zavaleta SchwartzNo ratings yet
- Ma DPH Covid-19 DashboardDocument17 pagesMa DPH Covid-19 DashboardRami Abou-SabeNo ratings yet
- Ma DPH Covid-19 DashboardDocument17 pagesMa DPH Covid-19 DashboardJohn WallerNo ratings yet
- Al Warsan - DWTC Holding AreaDocument3 pagesAl Warsan - DWTC Holding AreamyfaithnkaNo ratings yet
- Daily production report for Las Bambas mineDocument1 pageDaily production report for Las Bambas minehenry gavancho cutiNo ratings yet
- Caramat Words Periodic TableDocument1 pageCaramat Words Periodic TableJude Arvie Raya CaramatNo ratings yet
- Fat Soluble Vitamin - ADocument5 pagesFat Soluble Vitamin - AShiv RammNo ratings yet
- Table ViewDocument1 pageTable ViewÂßdÛl KarÌmNo ratings yet
- Hydrogen Power Handbook (3206)Document34 pagesHydrogen Power Handbook (3206)cesar baranda100% (2)
- Continuous Neutralising Section PDFDocument1 pageContinuous Neutralising Section PDFRodrigo Ratto TiburcioNo ratings yet
- Other Fluids CharacteristicsDocument1 pageOther Fluids CharacteristicsKrishna PATELNo ratings yet
- World's Busiest Airports 2018Document1 pageWorld's Busiest Airports 2018fundeyNo ratings yet
- San Francisco Bay Area MapDocument1 pageSan Francisco Bay Area Mapmareymorsy2822No ratings yet
- Factsheet GCP Pattaya enDocument2 pagesFactsheet GCP Pattaya enOon KooNo ratings yet
- RFCC units maximize propylene production from heavy residual feedstocksDocument11 pagesRFCC units maximize propylene production from heavy residual feedstocksNguyễn Thành Tài100% (1)
- AM SirrDocument218 pagesAM SirrTrash SinghNo ratings yet
- NORTE AMERICA Argus Fertilizer North America Map 2019Document1 pageNORTE AMERICA Argus Fertilizer North America Map 2019rubenpeNo ratings yet
- Oil Companies TransitoinDocument1 pageOil Companies TransitoinandresNo ratings yet
- Plant and Machinery (1) - 3Document1 pagePlant and Machinery (1) - 3SM AreaNo ratings yet
- Ma DPH Covid-19 DashboardDocument17 pagesMa DPH Covid-19 DashboardRami Abou-SabeNo ratings yet
- PID Ver 1Document1 pagePID Ver 1Gillian AmbaNo ratings yet
- 1 Map 20180109 f61424dbDocument1 page1 Map 20180109 f61424dbkumar.arasu8717No ratings yet
- Serial Write and Read Loop: Bytes at PortDocument1 pageSerial Write and Read Loop: Bytes at PortErikChinachiAmánNo ratings yet
- Ma DPH Covid-19 DashboardDocument17 pagesMa DPH Covid-19 DashboardRami Abou-SabeNo ratings yet
- Ma DPH Covid-19 DashboardDocument17 pagesMa DPH Covid-19 DashboardRami Abou-SabeNo ratings yet
- Chemical Resistance Guide: Industrial ChemicalsDocument4 pagesChemical Resistance Guide: Industrial ChemicalsMohamed TallyNo ratings yet
- AP Physics Free Response Practice – Dynamics – ANSWERSDocument8 pagesAP Physics Free Response Practice – Dynamics – ANSWERSasmaa eNo ratings yet
- 3b-Torque FR Practice ProblemsDocument4 pages3b-Torque FR Practice ProblemsSnowy JiangNo ratings yet
- Great Neck South High SchoolDocument12 pagesGreat Neck South High Schoolasmaa eNo ratings yet
- AP Physics Free Response Practice – Work Power EnergyDocument31 pagesAP Physics Free Response Practice – Work Power EnergyutheinsweNo ratings yet
- 4a Wep MCDocument14 pages4a Wep MCasmaa eNo ratings yet
- AP Physics Multiple Choice PracticeDocument5 pagesAP Physics Multiple Choice Practiceasmaa eNo ratings yet
- Lit Comp Questions (Set 1)Document5 pagesLit Comp Questions (Set 1)asmaa eNo ratings yet
- Chemical Equilibrium .PresentationDocument17 pagesChemical Equilibrium .PresentationtalhawasimNo ratings yet
- Introduction To Electrochemistry: Kimia Analitik Universitas PertaminaDocument21 pagesIntroduction To Electrochemistry: Kimia Analitik Universitas PertaminaVincentius EkyNo ratings yet
- ARTIKEL PDF - Nurannisa Chandra DewiDocument40 pagesARTIKEL PDF - Nurannisa Chandra DewiYanski DarmantoNo ratings yet
- Cap4 (Imperfections in The Atomic Arrangement)Document15 pagesCap4 (Imperfections in The Atomic Arrangement)Azalia TovarNo ratings yet
- Aoac 983.13Document1 pageAoac 983.13Chemist İnançNo ratings yet
- Introduction To PH PDFDocument3 pagesIntroduction To PH PDFRichard ObinnaNo ratings yet
- Crystallography Point GroupsDocument10 pagesCrystallography Point GroupsSireesh Babu MaddinediNo ratings yet
- Installing The Agilent 6890 Oven Insert For Fast ChromatographyDocument6 pagesInstalling The Agilent 6890 Oven Insert For Fast Chromatography신지훈No ratings yet
- MSCCH 505LDocument121 pagesMSCCH 505LVishnu ShankerNo ratings yet
- Crystal Structures IIDocument35 pagesCrystal Structures IISamNo ratings yet
- Flame Photometry PDFDocument9 pagesFlame Photometry PDFHina AftabNo ratings yet
- Snamprogetti New MTBE Production DesignDocument6 pagesSnamprogetti New MTBE Production DesignViệt HàNo ratings yet
- First Set Ee Lab Viva Questions by Sai Harsha & Suresh SirDocument3 pagesFirst Set Ee Lab Viva Questions by Sai Harsha & Suresh SirAJAYNo ratings yet
- Isolation of ProteinDocument6 pagesIsolation of ProteinGrace AquinoNo ratings yet
- Practice Test Acids BasesDocument4 pagesPractice Test Acids Basesdemetri lanezNo ratings yet
- Seven Crystal Systems and Miller IndicesDocument21 pagesSeven Crystal Systems and Miller Indicesmithunesh 07No ratings yet
- Ee Lab ExamDocument16 pagesEe Lab ExamjyothisunilabrahamNo ratings yet
- Measurement of Acid Neutralizing Capacity: ANC (HCO) 2 (CO) (OH) - (H)Document8 pagesMeasurement of Acid Neutralizing Capacity: ANC (HCO) 2 (CO) (OH) - (H)rio kurniawanNo ratings yet
- A + B A + B: Mtchem3: Analytical Chemistry For MlsDocument7 pagesA + B A + B: Mtchem3: Analytical Chemistry For MlsJhona Mae CortesNo ratings yet
- Redox TitrationDocument6 pagesRedox TitrationMohammad RussellNo ratings yet
- MRES216 Physical Techniques For The Study of Biological SystemsDocument10 pagesMRES216 Physical Techniques For The Study of Biological SystemsSaurabh ShineNo ratings yet
- QuinineDocument9 pagesQuinineAhmad AlbabNo ratings yet
- Column and Thin Layer ChromatographyDocument3 pagesColumn and Thin Layer ChromatographyAileen Delos SantosNo ratings yet
- What is pH? Understanding Acidic and Basic SolutionsDocument18 pagesWhat is pH? Understanding Acidic and Basic SolutionsDanielle GunterNo ratings yet
- Solubility and ExtractionDocument3 pagesSolubility and ExtractionDaniel McDermottNo ratings yet
- MBR Membrane MBR Membrane Specs & UsesDocument10 pagesMBR Membrane MBR Membrane Specs & UsesdeddyNo ratings yet
- ISE Direct Vs Indirect Ica v2-bdb72478Document27 pagesISE Direct Vs Indirect Ica v2-bdb72478Usman AbbasNo ratings yet
- SEPARATION METHODS GUIDEDocument58 pagesSEPARATION METHODS GUIDESandi Mahesa0% (1)