Professional Documents
Culture Documents
CGMP Tree
Uploaded by
v9991 v9991Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CGMP Tree
Uploaded by
v9991 v9991Copyright:
Available Formats
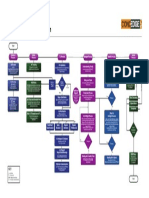
GOOD MANUFACTURING PRACTICES IN PHARMA INDUSTRY
cGMP TREE
CVMP
Kiran Kota
Design, Build & maintain
facilities & Equipment
Uncover the facts GMP Guidelines Incident Investigation Change management
Complaints, Recall
GMP as per Schedule "M" management
(www.cdsco.nic.in)
Validate the work Be competent Product quality review
GMP as per WHO
(www.who.int) Self Inspection
Document the work 10 Commandments GMP as per MCA now known as MHRA Quality management
(www.mca.gov.uk)
of GMP GMP as per TGA system
Integrate quality in all
(www.tga.gov.au) Vendor Qualification
works / areas
Follow the written GMP as per US FDA
procedure (www.fda.gov)
GMP as per ICH guidelines Risk Assessment Audit for Compliance Batch Release
(www.ich.org)
Write Procedures Be clean Maintain Data Integrity
Personnel must aware
Organization Chart
Area restricted to authorized GMP/GLP
personnel
Reconciliation of the In-process controls & Regular trainings & Its
Sufficient Qualified Personnel
product Environmental controls effectiveness check
cGMP
Clearly defined
Performed & supervised by Production Personnel responsibilities
competent people
Personnel Hygiene
Unidirectional Flow
Medical Examination prior to
Avoid mix up & cross recruitment Use protective
contamination garments
Validation of Critical
Yields should be monitored &
steps
should be within limit
Quality Control
Maintained as per requirement
Document Traceability Laboratory reagents, Volumetric
Procedure for all
activities solutions, reference standards and
Culture media- as per procedure Regular monitoring of Environmental
Adequate Size
conditions
System to retrieve superseded
documents Approved, signed and dated by Testing of all Materials as per Validated & Approved Analytical
authorized procedure Methods Sufficient place for
warehousing
Qualification &
Only current version to Calibration
be in used Mfg / Testing records, Log Premises &
Documentation books, Line clearance records,
Flow in logical order
protocol & reports etc. Equipment
Online documentation for all Properly labelled & Store as per
activities requirements
Unambiguous contents
Pipe work with flow
directions Separate room for sensitive
Electronically stored-Protected Reproduced documents-clear Instruments/ Materials
by backup support and legible
Contact parts must not be reactive,
additive or absorptive Easy to Clean
A good GMP document should be Accurate, Clear, Complete, Consistent, Indelible, Legible, Timely, Direct, Authentic and Authorized.
You might also like
- Industrial Pharmacy: GMP For Pharmaceutical ProductsDocument11 pagesIndustrial Pharmacy: GMP For Pharmaceutical ProductsIntn LestariNo ratings yet
- Contoh Proses Bisnis: Management ProcessDocument2 pagesContoh Proses Bisnis: Management ProcessSilvi KhoNo ratings yet
- Industrial Pharmacy 2020-2Document12 pagesIndustrial Pharmacy 2020-2Alexander KwaitotaNo ratings yet
- Attachment - FSMS Process Interaction (Rev 0) - 210901Document1 pageAttachment - FSMS Process Interaction (Rev 0) - 210901QA HBTNo ratings yet
- Process Interaction DiagramDocument1 pageProcess Interaction DiagramPooja SankhlaNo ratings yet
- Presentation EQMSDocument13 pagesPresentation EQMSmohamed waleed fouadNo ratings yet
- Quality Management Maturity - UsfdaDocument1 pageQuality Management Maturity - Usfdavijay maliNo ratings yet
- PRP ISTS 22002-1 2009 - Updated 2019 Shared PDFDocument77 pagesPRP ISTS 22002-1 2009 - Updated 2019 Shared PDFJill DagreatNo ratings yet
- Process MapDocument4 pagesProcess Mapkmvimal36No ratings yet
- OCP Framework - Mindmap.2019v1.4Document1 pageOCP Framework - Mindmap.2019v1.4LCNo ratings yet
- Communication Skills Jan2008Document28 pagesCommunication Skills Jan2008Bosse BoseNo ratings yet
- Storage Environments in The Pharmaceutical Industry: Monitoring Manufacturing, Production andDocument8 pagesStorage Environments in The Pharmaceutical Industry: Monitoring Manufacturing, Production andMohamed ZhranNo ratings yet
- Q9 Executive SummaryDocument16 pagesQ9 Executive SummaryNarendrakumarNo ratings yet
- API Drug Manufacturing Services and ProcessDocument3 pagesAPI Drug Manufacturing Services and ProcessAadityaRoyNo ratings yet
- RMCDocument3 pagesRMCsarav10No ratings yet
- Process Analysis: SL No Process Proces S Owner Input Output Methods Interfaces With Measure of Performance (MOP)Document12 pagesProcess Analysis: SL No Process Proces S Owner Input Output Methods Interfaces With Measure of Performance (MOP)DhinakaranNo ratings yet
- Quality Regulatory Global Pharmaceutical: and Scientific Services IndustryDocument8 pagesQuality Regulatory Global Pharmaceutical: and Scientific Services Industrykrutik shahNo ratings yet
- Module - 5 - PPPQMS - Lifecycle Management 2018Document11 pagesModule - 5 - PPPQMS - Lifecycle Management 2018Indra Maghfurin100% (1)
- Gestión de No Conformidad (2QN) - Diagramas de ProcesoDocument2 pagesGestión de No Conformidad (2QN) - Diagramas de ProcesoDiego CincottaNo ratings yet
- Fcaam - Organic Agriculture Production NC 11 - Organic Concoctions and ExtractsDocument3 pagesFcaam - Organic Agriculture Production NC 11 - Organic Concoctions and ExtractsLANY T. CATAMINNo ratings yet
- Good Manufacturing Practices For Pharmaceutical Products: Fakultas Farmasi Universitas Pancasila Jakarta, 2018Document54 pagesGood Manufacturing Practices For Pharmaceutical Products: Fakultas Farmasi Universitas Pancasila Jakarta, 2018Ayu LestaryNo ratings yet
- Sequence&Process INTERACTION LpiDocument1 pageSequence&Process INTERACTION LpiEmad EmadNo ratings yet
- Process Chart: Risk Management Approach To Vendor PerformanceDocument1 pageProcess Chart: Risk Management Approach To Vendor PerformanceHà YênNo ratings yet
- Temu 13. Manajemen Mutu Pangan TerpaduDocument17 pagesTemu 13. Manajemen Mutu Pangan Terpadunatasya amabelNo ratings yet
- 03 DWM Master - DWM - WHAT & WHYDocument12 pages03 DWM Master - DWM - WHAT & WHYVenuNo ratings yet
- 18 Management ReviewDocument1 page18 Management Reviewkumarnitesh173No ratings yet
- Guiding Principles For Plant Quality - 20140708Document4 pagesGuiding Principles For Plant Quality - 20140708didik dadtNo ratings yet
- Roadmap For ActivitiesDocument1 pageRoadmap For ActivitiesaslamNo ratings yet
- Quality & Industrial Performance: Managing ChangeDocument37 pagesQuality & Industrial Performance: Managing ChangeMojtaba MousaviNo ratings yet
- ISO CalibrationDocument3 pagesISO Calibrationnoufal27No ratings yet
- 2.seamless Integration Daniel NilssonDocument20 pages2.seamless Integration Daniel Nilssonleon tagoreNo ratings yet
- Design of A Process Qualification and Continued Process Verification Program Within An Enhanced Development FrameworkDocument20 pagesDesign of A Process Qualification and Continued Process Verification Program Within An Enhanced Development Frameworkschumon100% (1)
- 4 M Change LatestDocument8 pages4 M Change LatestVikas KashyapNo ratings yet
- Advantages of A Tree Structure FMEA Agility Scalability and Accelerated Quality FeedbackDocument6 pagesAdvantages of A Tree Structure FMEA Agility Scalability and Accelerated Quality Feedbackchemikas8389No ratings yet
- Quality Handbook: October 2018 Semiconductor Samsung Electronics Co., LTDDocument21 pagesQuality Handbook: October 2018 Semiconductor Samsung Electronics Co., LTDtantibaNo ratings yet
- Newspaper Process FlowDocument1 pageNewspaper Process FlowManish BaptistNo ratings yet
- Process Interaction MatrixDocument1 pageProcess Interaction Matrixشیخ صادقNo ratings yet
- Process MapDocument1 pageProcess MapqualityNo ratings yet
- Change ControlDocument62 pagesChange ControlshivanagiriNo ratings yet
- Milk Vending Machines Quality Products at Competitive Prices Sub-BrandingDocument1 pageMilk Vending Machines Quality Products at Competitive Prices Sub-BrandingRobin GhotiaNo ratings yet
- Ejemplos Tortuga IatfDocument1 pageEjemplos Tortuga IatfAnonymous XzqXVMjNo ratings yet
- Pharmaceutical CompaniesDocument6 pagesPharmaceutical CompaniesMohammad Mostakim- Al-RashidNo ratings yet
- Sap Pharma DemoDocument36 pagesSap Pharma DemoAnandKumar S100% (1)
- Risk & Opportunities MatrixDocument7 pagesRisk & Opportunities MatrixAmit AnandNo ratings yet
- Production Part ApprovalDocument3 pagesProduction Part Approvalvidya410gmailcomNo ratings yet
- Quality Improvement Strategy FlowchartDocument3 pagesQuality Improvement Strategy FlowchartDavid GeorgeNo ratings yet
- PQR Overview 2016Document24 pagesPQR Overview 2016ike mayaNo ratings yet
- Pharma Questions and Answers (Biopharmaceutics)Document5 pagesPharma Questions and Answers (Biopharmaceutics)Piyush RajNo ratings yet
- TEF - Awareness - Mining ForumDocument18 pagesTEF - Awareness - Mining ForumTC BengalonNo ratings yet
- MFM Annual Report (47 196)Document151 pagesMFM Annual Report (47 196)Char YosNo ratings yet
- GM-GMS Operating Guideline 13.0 In-Process Control and VerificationDocument55 pagesGM-GMS Operating Guideline 13.0 In-Process Control and VerificationSalaNo ratings yet
- Statistical Quality ControlDocument30 pagesStatistical Quality ControlSANDIP KUMARNo ratings yet
- Prereq Verification TableDocument11 pagesPrereq Verification Tablealias brownNo ratings yet
- Ubject: PFMEA Level Up: Quality Marshal Training & WorkshopDocument38 pagesUbject: PFMEA Level Up: Quality Marshal Training & WorkshopAditya vatsyayanNo ratings yet
- 8.0 Quality Process MapDocument1 page8.0 Quality Process MapRohit SoniNo ratings yet
- TMap NEXT Poster (EN) PDFDocument1 pageTMap NEXT Poster (EN) PDFJose Luis Becerril BurgosNo ratings yet
- ISPE CCChPlantFacilitiesEngPharmaIndDocument28 pagesISPE CCChPlantFacilitiesEngPharmaIndHamidNo ratings yet
- Master Sheet Process Audit Check SheetDocument11 pagesMaster Sheet Process Audit Check SheetRakesh S100% (1)
- Supplier System Audit Checklist - Action Plan (Updated As On 08.11.21)Document9 pagesSupplier System Audit Checklist - Action Plan (Updated As On 08.11.21)HR BGHNo ratings yet
- Start UpsDocument6 pagesStart Upsv9991 v9991No ratings yet
- Confidence InfographicDocument12 pagesConfidence Infographicv9991 v9991No ratings yet
- 100 KPIsDocument101 pages100 KPIsv9991 v9991No ratings yet
- Csa CSV LNDocument4 pagesCsa CSV LNv9991 v9991No ratings yet
- Chat GPT Di Audit Manual and Electronic DataDocument2 pagesChat GPT Di Audit Manual and Electronic Datav9991 v9991No ratings yet
- Csa CSV Change 1Document5 pagesCsa CSV Change 1v9991 v9991No ratings yet
- Differences Between Csa CSVDocument5 pagesDifferences Between Csa CSVv9991 v9991No ratings yet
- Business ModelsDocument32 pagesBusiness Modelsv9991 v9991No ratings yet
- Stem Cell RegistrationDocument5 pagesStem Cell Registrationv9991 v9991No ratings yet
- Saas Validation StrategyDocument10 pagesSaas Validation Strategyv9991 v9991No ratings yet
- Annexure To URS - Spreadsheet ValidationDocument1 pageAnnexure To URS - Spreadsheet Validationv9991 v9991No ratings yet
- ViewDocument8 pagesViewv9991 v9991No ratings yet
- Induct Program SCHDDocument1 pageInduct Program SCHDv9991 v9991No ratings yet
- Ich Terms and DefinitionsDocument197 pagesIch Terms and Definitionsv9991 v9991No ratings yet
- SuccessDocument16 pagesSuccessv9991 v9991No ratings yet
- SuccessDocument11 pagesSuccessv9991 v9991No ratings yet
- ThinkDocument11 pagesThinkv9991 v9991No ratings yet
- Success ValuesDocument16 pagesSuccess Valuesv9991 v9991No ratings yet
- Pithampur, India 03-21 Through 29-2023 - 483 PDFDocument13 pagesPithampur, India 03-21 Through 29-2023 - 483 PDFv9991 v9991No ratings yet
- Feedback - Quality by Design For Development Services (1-23)Document9 pagesFeedback - Quality by Design For Development Services (1-23)v9991 v9991No ratings yet
- Induct Program SCHDDocument1 pageInduct Program SCHDv9991 v9991No ratings yet
- Infographic Enablers 6Document15 pagesInfographic Enablers 6v9991 v9991No ratings yet
- Practice Problems (Comprehensive)Document12 pagesPractice Problems (Comprehensive)The Brain Dump PHNo ratings yet
- Ticker LTP O H L Change % Change OpenDocument22 pagesTicker LTP O H L Change % Change OpenPrasanna PharaohNo ratings yet
- 024-Industrial Refractories Corporation of The Philippines vs. CA, Et Al G.R. No. 122174 October 3, 2002Document5 pages024-Industrial Refractories Corporation of The Philippines vs. CA, Et Al G.R. No. 122174 October 3, 2002wewNo ratings yet
- 9 Bonus and Right IssueDocument4 pages9 Bonus and Right IssueRohith KumarNo ratings yet
- Unit 1 Calculation of Tax LiabilityDocument10 pagesUnit 1 Calculation of Tax LiabilitySupreet UdarNo ratings yet
- Gender Neutral Toys DebateDocument3 pagesGender Neutral Toys Debateapi-528782034No ratings yet
- PMBOK 7th Edition (iBIMOne - Com) - ENG-11Document5 pagesPMBOK 7th Edition (iBIMOne - Com) - ENG-11Felipe Guimaraes PazinNo ratings yet
- Essential Leadership Lessons From Top CEOs - Lou Gerstner, Jack Welch, Sam Walton, Howard Hughes, Lee Iacocca, Phil Knight, Walt Disney, Carlos Ghosn, Andrew S.Grove PDFDocument112 pagesEssential Leadership Lessons From Top CEOs - Lou Gerstner, Jack Welch, Sam Walton, Howard Hughes, Lee Iacocca, Phil Knight, Walt Disney, Carlos Ghosn, Andrew S.Grove PDFananth080864100% (2)
- Cs7E6 - Software Quality Assurance: Pre-RequisiteDocument2 pagesCs7E6 - Software Quality Assurance: Pre-Requisitesakthi VelNo ratings yet
- Impact of GST On Food and Hospitality IndustryDocument98 pagesImpact of GST On Food and Hospitality IndustrySimran JaiswalNo ratings yet
- Asse Ssment of Supply Chain Practice in Case of Bahir Dar Leather TanneryDocument44 pagesAsse Ssment of Supply Chain Practice in Case of Bahir Dar Leather TanneryÄbřîśh Łìj MęŘãNo ratings yet
- Cecchetti 5e Chapter02Document45 pagesCecchetti 5e Chapter02Gail Marie VasquezNo ratings yet
- FTF 2023-02-04 1675555956991Document3 pagesFTF 2023-02-04 1675555956991Brayan Teodoro Santiago Pascual100% (4)
- Schwab Family ValuesDocument18 pagesSchwab Family ValuesguingooNo ratings yet
- Georgia Power Utility AssistanceDocument2 pagesGeorgia Power Utility AssistanceABC15 NewsNo ratings yet
- Implementation of Lean Principles: Towards Augmenting Productivity in Construction Industry - A Literature ReviewDocument3 pagesImplementation of Lean Principles: Towards Augmenting Productivity in Construction Industry - A Literature ReviewAce NovoNo ratings yet
- WEST COAST COLL-WPS OfficeDocument6 pagesWEST COAST COLL-WPS OfficeJan Kryz Marfil PalenciaNo ratings yet
- Telecom Sector Porter's 5 Force AnalysisDocument3 pagesTelecom Sector Porter's 5 Force AnalysisKARTIK ANAND100% (1)
- Name Change RequestDocument2 pagesName Change RequestRonald TorresNo ratings yet
- 661 - Gandhi DarshanDocument5 pages661 - Gandhi DarshanrtiNo ratings yet
- Small Business ManagementDocument77 pagesSmall Business ManagementParamiedu Finance100% (1)
- ACCTG 4 Financial Accounting Theory and Practice Part 2: Lyceum-Northwestern UniversityDocument4 pagesACCTG 4 Financial Accounting Theory and Practice Part 2: Lyceum-Northwestern UniversityAmie Jane MirandaNo ratings yet
- Public Sector Marketing: Department of Public Administration Fatima Jinnah Women University Instructor: Sana MukarramDocument22 pagesPublic Sector Marketing: Department of Public Administration Fatima Jinnah Women University Instructor: Sana MukarramWaljia BaigNo ratings yet
- Corporate Objectives, Strategy and Structure: Prepared By: Ms. Nelda A. Rosima InstructorDocument27 pagesCorporate Objectives, Strategy and Structure: Prepared By: Ms. Nelda A. Rosima InstructorValerie Kaye FamilaranNo ratings yet
- Brochure 2023Document3 pagesBrochure 2023Sandeep SNo ratings yet
- RR PLC Annual Report 2022Document164 pagesRR PLC Annual Report 2022Mann AgrawalNo ratings yet
- Allama Iqbal Open University, Islamabad: (Department of Commerce)Document5 pagesAllama Iqbal Open University, Islamabad: (Department of Commerce)Sohail Liaqat AliNo ratings yet
- Gujarat Alkalies and Chemical Init Mar 19 IndiaDocument15 pagesGujarat Alkalies and Chemical Init Mar 19 IndiaDave LiNo ratings yet
- LicensesDocument3 pagesLicensesВасилий КоробкаNo ratings yet
- Parental Leave Application Form - HR 108 (J)Document3 pagesParental Leave Application Form - HR 108 (J)LexNo ratings yet