Professional Documents
Culture Documents
q4 Act 4 Gay Lussac Law
q4 Act 4 Gay Lussac Law
Uploaded by
Yoji Delo santosOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
q4 Act 4 Gay Lussac Law
q4 Act 4 Gay Lussac Law
Uploaded by
Yoji Delo santosCopyright:
Available Formats
Name: ________________________________ Sec/Grp. No.
_________________Score: ________
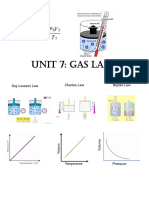
ACTIVITY NO. 4
Gay-Lussac Law
A. Observe and analyze the illustration below and complete the information needed on
the Table 1.
A. Table 1. Pressure and Temperature B. Plot the data on the graph with
Relationship Pressure at the Y-axis and
temperature on the X-axis.
TRIAL PRESSURE TEMPERATURE
0C K
Guide Questions:
Q1. Describe what happens to the pressure of gases in the container as temperature
increases?
Q2. Describe what happens to the temperature from the left to the right container?
Q3. Describe the graph.
Q4. What is the relationship between the pressure and temperature of gases at constant
volume?
You might also like
- Gas Laws Graphing ActivityDocument2 pagesGas Laws Graphing Activityapi-213793181No ratings yet
- Boyle's & Charles' Law WorksheetDocument6 pagesBoyle's & Charles' Law WorksheetMary Grace Jerna Artazo Nozal-CuadraNo ratings yet
- Properties of SteamDocument83 pagesProperties of Steama_storm100% (7)
- Cecilia Guzman - IdealGasLawSE GizmosDocument8 pagesCecilia Guzman - IdealGasLawSE GizmosCecilia Guzman100% (1)
- Charles LawDocument8 pagesCharles LawasilahrafarNo ratings yet
- Boy Les Charles SeDocument7 pagesBoy Les Charles SeDania McleanNo ratings yet
- Demo PrintDocument4 pagesDemo Printkimmymantos022No ratings yet
- Demo PrintDocument4 pagesDemo Printkimmymantos022No ratings yet
- Activity 5 Science 10 4th QDocument2 pagesActivity 5 Science 10 4th QStarskie MonacarNo ratings yet
- Gas Laws WeberciseDocument7 pagesGas Laws Weberciseapi-3652150540% (1)
- Pre-Assessment Directions: Answer The Following Questions Below About Volume-Pressure Relationship and Write Your Answer inDocument2 pagesPre-Assessment Directions: Answer The Following Questions Below About Volume-Pressure Relationship and Write Your Answer inMa'am MercadoNo ratings yet
- Activity Science 10 Boyles LawDocument2 pagesActivity Science 10 Boyles LawirishcatherinealbisNo ratings yet
- Simulation Gaslaws StudentDocument8 pagesSimulation Gaslaws StudentSerenity WadeNo ratings yet
- States of Matter Worksheet - TaggedDocument6 pagesStates of Matter Worksheet - Taggedguiang.michaelaNo ratings yet
- 4Q Sci10 Las2 - Charles' LawDocument4 pages4Q Sci10 Las2 - Charles' Lawrectoann08No ratings yet
- Science 10 Q4 Module 1Document29 pagesScience 10 Q4 Module 1Maki Tuna100% (2)
- S10MT IIj 20 CHARLES LAW ABUEVA R1Document2 pagesS10MT IIj 20 CHARLES LAW ABUEVA R1Leil RiegoNo ratings yet
- Science 10 Las 4-1Document5 pagesScience 10 Las 4-1Michael TuyayNo ratings yet
- Student Exploration: Boyle's Law and Charles's LawDocument14 pagesStudent Exploration: Boyle's Law and Charles's LawStephanie ValienteNo ratings yet
- Unit 7 Gas Laws Packet 2021Document40 pagesUnit 7 Gas Laws Packet 2021Alberto Laborte Jr.No ratings yet
- Student Exploration: Boyle's Law and Charles' LawDocument8 pagesStudent Exploration: Boyle's Law and Charles' LawjzbNlka0% (1)
- Boy Les Charles SeDocument14 pagesBoy Les Charles SeLenny Morell ZayasNo ratings yet
- Part 1: Boyle's Law: Pressure-vs-Volume: MaterialsDocument5 pagesPart 1: Boyle's Law: Pressure-vs-Volume: MaterialsMadison IngramNo ratings yet
- States of Matter - Charles GuiangDocument6 pagesStates of Matter - Charles Guiangguiang.michaelaNo ratings yet
- W2 CharlessDocument2 pagesW2 Charlessalexandria iskandarNo ratings yet
- GC1 Q1 Week-5ab PDFDocument12 pagesGC1 Q1 Week-5ab PDFLovely MavilNo ratings yet
- W3 Guy-LussacDocument2 pagesW3 Guy-Lussacalexandria iskandarNo ratings yet
- Student Exploration: Boyle's Law and Charles's LawDocument12 pagesStudent Exploration: Boyle's Law and Charles's LawMF - 11AK 827776 Central Peel SSNo ratings yet
- Charles LawDocument3 pagesCharles Lawruthangeladizon.13No ratings yet
- Pressure and TemperatureDocument2 pagesPressure and TemperatureTanish bossNo ratings yet
- Student Exploration: Boyle's Law and Charles' LawDocument7 pagesStudent Exploration: Boyle's Law and Charles' Lawapi-280439402No ratings yet
- Boyles & Charles' Law Simulation GizmoDocument7 pagesBoyles & Charles' Law Simulation GizmoamyNo ratings yet
- SCIENCE10 Q4W1 v2Document16 pagesSCIENCE10 Q4W1 v2Trixie EuniceNo ratings yet
- Phys 23 T2 The Constant Volume Air ThermometerDocument4 pagesPhys 23 T2 The Constant Volume Air ThermometerShashwat ChakrabortiNo ratings yet
- Student Exploration: Boyle's Law and Charles's LawDocument5 pagesStudent Exploration: Boyle's Law and Charles's LawMuranoNo ratings yet
- Topic 7 Practice PacketDocument46 pagesTopic 7 Practice Packetsg 85No ratings yet
- Formative To PrintDocument1 pageFormative To PrintshellaineNo ratings yet
- W1 BoyleDocument2 pagesW1 Boylealexandria iskandarNo ratings yet
- Heating Curve WorksheetDocument3 pagesHeating Curve Worksheetdhruvin.prasanthNo ratings yet
- Science 10 Q4 LAS Week 1.2Document4 pagesScience 10 Q4 LAS Week 1.2Eainne David DocogNo ratings yet
- Lab #52: Pressure and Volume Relationship in Gases-Boyle's LawDocument3 pagesLab #52: Pressure and Volume Relationship in Gases-Boyle's LawSidemen For LifeNo ratings yet
- Experiment: Ideal Gas Law (Manual+ Worksheet) : UsingDocument7 pagesExperiment: Ideal Gas Law (Manual+ Worksheet) : UsingMazanda YalinduaNo ratings yet
- Physical Chemistry Mid Term ExamDocument4 pagesPhysical Chemistry Mid Term ExamMaricar DimasNo ratings yet
- Phase DiagramDocument3 pagesPhase DiagramTing TCNo ratings yet
- RobieDocument231 pagesRobiegroovyplayerNo ratings yet
- Change of State of Gazes 1Document7 pagesChange of State of Gazes 1nadjimohamedasaad45No ratings yet
- Gay Lussac's Law Web 01-02Document3 pagesGay Lussac's Law Web 01-02Kevin Rick SantosNo ratings yet
- Boyle'S Law and Charles' Law: Learning Activity Sheets Grade 10 - ScienceDocument4 pagesBoyle'S Law and Charles' Law: Learning Activity Sheets Grade 10 - ScienceCristina Yuson0% (1)
- Boyle'S Law and Charles' Law: Learning Activity Sheets Grade 10 - ScienceDocument4 pagesBoyle'S Law and Charles' Law: Learning Activity Sheets Grade 10 - ScienceCristina Yuson67% (6)
- Robie and Hemingway PDFDocument464 pagesRobie and Hemingway PDFtermmasterNo ratings yet
- Rate and Extent of Chemical Reactions Revision Test 2Document3 pagesRate and Extent of Chemical Reactions Revision Test 2KiaNo ratings yet
- Charle's LawDocument5 pagesCharle's Lawdark iceNo ratings yet
- IdealGasLawSEDocument6 pagesIdealGasLawSENada DawoodNo ratings yet
- Gas Law ActivityDocument7 pagesGas Law Activitydaniel tabuzoNo ratings yet
- Gay-Lussac's Law ACT-SHEETDocument1 pageGay-Lussac's Law ACT-SHEETR4A, QUEZON, BRICENIO, AIDANN JOSEPH V., LNCHSNo ratings yet
- Mathematics LasDocument7 pagesMathematics LasAna Karen Balili-Velasco LabradorNo ratings yet
- Multiple Choice: Choose The Letter of The Best Answer.: B. Inversely ProportionalDocument7 pagesMultiple Choice: Choose The Letter of The Best Answer.: B. Inversely ProportionalClarence Mike BorjaNo ratings yet
- Lab - Pressure and TemperatureDocument4 pagesLab - Pressure and Temperatureapi-383619824No ratings yet
- Phase Equilibrium in Mixtures: International Series of Monographs in Chemical EngineeringFrom EverandPhase Equilibrium in Mixtures: International Series of Monographs in Chemical EngineeringNo ratings yet