Professional Documents
Culture Documents
Inherited B-Cell Dysfunction in Lean Individuals With Type 2 Diabetes
Uploaded by
Adam TorrensOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Inherited B-Cell Dysfunction in Lean Individuals With Type 2 Diabetes
Uploaded by
Adam TorrensCopyright:
Available Formats
COMMENTARY
Inherited b-Cell Dysfunction in Lean Individuals With
Type 2 Diabetes
Anne E.W. Mathews1 and Clayton E. Mathews2
Studies to identify the genes contributing to type 2 di-
abetes in lean individuals identified a region on chromo-
T
he syndromes comprising diabetes mellitus share some 21q (12). Follow-up examination of this region led to
the common feature of b-cell failure. Inheritance the identification of KCNJ15 (potassium inwardly-rectifying
of b-cell failure is clear in patients diagnosed channel, subfamily J, member 15) as a susceptibility gene
with the MODY (maturity-onset diabetes of the (13). KCNJ15, also known as Kir4.2, is expressed in
young, also referred to as monogenic diabetes in youth) a wide variety of tissues and cells including peripheral
Downloaded from http://diabetesjournals.org/diabetes/article-pdf/61/7/1659/561354/1659.pdf by guest on 06 March 2023
syndromes (1), as well as in permanent neonatal diabetes blood, kidney, and pancreatic islet cells (13). The risk al-
mellitus (2) where mutations in genes essential for insulin lele mRNA is present at elevated levels due to increased
secretion associate with elevated blood glucose. Causative mRNA stability. Overexpression of KCNJ15 in the rat b-cell
alleles for b-cell dysfunction have been identified for the line INS1 resulted in a significant reduction in GSIS. These
monogenic forms of diabetes. Likewise, a role for genes data support a role for the KCNJ15 risk allele as causa-
impacting glucose-stimulated insulin secretion has been tive in b-cell dysfunction observed in lean type 2 diabetic
proposed for some polygenic forms of diabetes mellitus. patients.
Genome-wide association studies have not associated single In this issue of Diabetes, Okamoto et al. (8) examined
nucleotide polymorphisms (SNPs) in genes required for the mechanism whereby KCNJ15 inhibits GSIS using both
optimal insulin secretion with autoimmune type 1A dia- in vitro and in vivo approaches and made several impor-
betes. In contrast, latent autoimmune diabetes of adults tant observations. First, expression of KCNJ15 is posi-
has been associated with TCF7L2 (3), a gene associated tively regulated by glucose. Second, the expression of
with reduced insulin secretion (4). TCF7L2 is one of the KCNJ15 was higher in the pancreatic islets of patients
most significant loci for type 2 diabetes (5) and, along with with type 2 diabetes. Additionally, knockdown of KCNJ15
HNF1B/HNF4A (6) and KCNJ11 (7), tie genes involved in INS1 cells resulted in an approximate twofold increase
in b-cell dysfunction to risk for developing type 2 diabetes in GSIS. Likewise, using a small inhibitory RNA approach,
in overweight individuals. In this issue of Diabetes, a new the authors were able to convincingly improve GSIS in both
report (8) proposes that a recently identified type 2 diabetes euglycemic and diabetic mouse models through knock-
risk gene is linked to dysfunctional glucose-stimulated in- down of KCNJ15. However, it should be noted that while
sulin secretion (GSIS) in lean Japanese patients with type 2 the suppression of this gene in diabetic mice did increase
diabetes. insulin secretion, it did not fully correct blood glucose
Lean type 2 diabetes is highly prevalent in Japan where levels to within the normal range. Finally, it is reported
more than half of the patients with this condition are that KCNJ15 associates with calcium-sensing receptor in
considered normal weight (BMI ,25) (9,10). The con- the plasma membrane, and the authors proposed that this
trasting general clinical phenotypes of obese individuals interaction blocks the KCNJ15 activity allowing for stabi-
with type 2 diabetes compared with lean individuals with lization of “post-prandial” membrane potential.
type 2 diabetes have recently been detailed (9). In this Unlike the MODY syndromes in which a single genetic
report of 40 case subjects with a diabetes duration of 10–12 defect results in decreased insulin secretion, the patho-
years, patients had an average age of onset of 56 6 2 years, genesis of type 2 diabetes in normal-weight individuals
low BMI (21 kg/m2), HbA1c of 6.7 6 0.1%, and fasting blood likely requires the contribution of multiple genetic regions
glucose of 144 6 9 mg/dL. Diabetes in lean Japanese (12). The data by Okamoto et al. (8) provide an important
patients has been associated with a low level of fasting step forward in our understanding of b-cell function/
insulin (32 6 17 pmol/L) in addition to reduced peripheral dysfunction and offer a paradigm to study the singular
glucose uptake (2.3 6 0.6 mg/kg/min) and elevated endog- impact of a risk gene within the pathology of diabetes. Once
enous glucose production (13.8 6 1.8 mmol/kg/min) (11). a mechanistic role has been elucidated, this provides a
These data suggest that this disorder in normal-weight further platform to investigate interactions with other
individuals results from impaired insulin secretion and identified risk alleles. Moving toward expression of the
action. specific risk allele in human b-cells, primary or a cell line
that secretes insulin (such as the recently developed
From the 1Department of Food Science and Human Nutrition, University of EndoC-bH1 [14]), will provide a further understanding
Florida, Gainesville, Florida; and the 2Departments of Pathology, Immunol- of how a synonymous SNP modifies protein function in
ogy, and Laboratory Medicine, University of Florida, Gainesville, Florida. a human system. It should also be pointed out that many
Corresponding author: Clayton E. Mathews, clayton.mathews@pathology.ufl
.edu. of the SNPs associated with polygenic forms of dia-
DOI: 10.2337/db12-0373 betes are in noncoding regions or induce synonymous
Ó 2012 by the American Diabetes Association. Readers may use this article as changes. Therefore, these studies provide a model for
long as the work is properly cited, the use is educational and not for profit,
and the work is not altered. See http://creativecommons.org/licenses/by studying polymorphisms that impact protein activity or
-nc-nd/3.0/ for details. function but do not alter protein sequence or a promoter
See accompanying original article, p. 1734. region.
diabetes.diabetesjournals.org DIABETES, VOL. 61, JULY 2012 1659
COMMENTARY
In light of these new data (8), the authors suggest KCNJ15 3. Grant SF, Hakonarson H, Schwartz S. Can the genetics of type 1 and type 2
as a new pharmacological target for individuals with re- diabetes shed light on the genetics of latent autoimmune diabetes in
adults? Endocr Rev 2010;31:183–193
duced insulin secretion. KCNJ15 depletion or inhibition 4. da Silva Xavier G, Loder MK, McDonald A, et al. TCF7L2 regulates late
with a small molecule may be an attractive therapeutic in events in insulin secretion from pancreatic islet beta-cells. Diabetes 2009;
any system where GSIS is decreased. This is strongly 58:894–905
supported by the data provided by whole animal systems 5. Grant SF, Thorleifsson G, Reynisdottir I, et al. Variant of transcription
where knockdown of Kcnj15 led to elevated insulin se- factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet
cretion in mice without diabetes and in mice with diabetes 2006;38:320–323
6. Waters KM, Stram DO, Hassanein MT, et al. Consistent association of type
induced by a mutation in the insulin 2 (Ins2) gene, that is 2 diabetes risk variants found in Europeans in diverse racial and ethnic
presumably independent of Kcnj15. However, caution groups. PLoS Genet 2010;6:e1001078
should be exercised. KCNJ15 appears to have a major role 7. Tabara Y, Osawa H, Kawamoto R, et al. Replication study of candidate
in renal function (15,16), and diabetic patients represent genes associated with type 2 diabetes based on genome-wide screening.
a population at increased risk for renal failure (17). Diabetes 2009;58:493–498
In conclusion, Okamoto et al. (8) present compelling 8. Okamoto K, Iwasaki N, Doi K, et al. Inhibition of glucose-stimulated insulin
secretion by KCNJ15, a newly identified susceptibility gene for type 2
evidence that KCNJ15 plays a role in reduced insulin se- diabetes. Diabetes 2012;61:1734–1741
cretion and the C566T SNP is likely causative in type 2 9. Tanaka S, Honda M, Wu B, Kazumi T. Clinical features of normal weight
diabetes. However, further work is required for a system- Japanese patients with type 2 diabetes who had formerly been obese.
Downloaded from http://diabetesjournals.org/diabetes/article-pdf/61/7/1659/561354/1659.pdf by guest on 06 March 2023
atic understanding of how KCNJ15 blocks GSIS. J Atheroscler Thromb 2011;18:115–121
10. Prato SD, LaSalle J, Matthaei S, Bailey CJ; Global Partnership for Effective
Diabetes Management. Tailoring treatment to the individual in type 2 di-
ACKNOWLEDGMENTS abetes practical guidance from the Global Partnership for Effective Di-
This work was supported by grants from the National abetes Management. Int J Clin Pract 2010;64:295–304
Institutes of Health (DK074656) (C.E.M.) as well as funding 11. Takahara M, Kaneto H, Katakami N, Matsuoka TA, Matsuhisa M,
Shimomura I. Impaired suppression of endogenous glucose production
from the American Diabetes Association (C.E.M.), the
in lean Japanese patients with type 2 diabetes mellitus. Diabetes Res
Juvenile Diabetes Research Foundation (C.E.M.), and the Clin Pract 2011;93:e1–e2
Sebastian Family Endowment for Diabetes Research 12. Iwasaki N, Cox NJ, Wang YQ, et al. Mapping genes influencing type 2 di-
(C.E.M.). abetes risk and BMI in Japanese subjects. Diabetes 2003;52:209–213
No potential conflicts of interest relevant to this article 13. Okamoto K, Iwasaki N, Nishimura C, et al. Identification of KCNJ15 as
were reported. a susceptibility gene in Asian patients with type 2 diabetes mellitus. Am
J Hum Genet 2010;86:54–64
The authors thank Mr. Clive Wasserfall and Dr. Desmond
14. Ravassard P, Hazhouz Y, Pechberty S, et al. A genetically engineered hu-
Schatz for critical reading of the manuscript and valuable man pancreatic b cell line exhibiting glucose-inducible insulin secretion.
advice. J Clin Invest 2011;121:3589–3597
15. Sindic A, Huang C, Chen AP, et al. MUPP1 complexes renal K+ channels to
alter cell surface expression and whole cell currents. Am J Physiol Renal
REFERENCES Physiol 2009;297:F36–F45
1. Winter WE, Nakamura M, House DV. Monogenic diabetes mellitus in 16. Pessia M, Imbrici P, D’Adamo MC, Salvatore L, Tucker SJ. Differential pH
youth. The MODY syndromes. Endocrinol Metab Clin North Am 1999;28: sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by
765–785 heteropolymerisation with Kir5.1. J Physiol 2001;532:359–367
2. Gloyn AL, Pearson ER, Antcliff JF, et al. Activating mutations in the gene 17. Ueda H, Ishimura E, Shoji T, et al. Factors affecting progression of renal
encoding the ATP-sensitive potassium-channel subunit Kir6.2 and perma- failure in patients with type 2 diabetes. Diabetes Care 2003;26:1530–
nent neonatal diabetes. N Engl J Med 2004;350:1838–1849 1534
1660 DIABETES, VOL. 61, JULY 2012 diabetes.diabetesjournals.org
You might also like
- Genética y DM-2Document29 pagesGenética y DM-2MaredQuispeNo ratings yet
- Cruz 2008Document6 pagesCruz 2008ERICK GUMILANGNo ratings yet
- Ijem 122553Document6 pagesIjem 122553Munawwar SaukaniNo ratings yet
- Skeletal Muscle Insulin Resistance in Normoglycemic SubjectDocument7 pagesSkeletal Muscle Insulin Resistance in Normoglycemic SubjectMuhammad Yusuf HanifNo ratings yet
- Tieuduongloai 2Document16 pagesTieuduongloai 220128139No ratings yet
- Pharmacological Management of Type 2 Diabetes Mellitus: Rationale For Rational Use of InsulinDocument9 pagesPharmacological Management of Type 2 Diabetes Mellitus: Rationale For Rational Use of InsulinAditya Rachman Van Der ArjunaqueeNo ratings yet
- Maturity Onset Diabetes of The Young: Review ArticleDocument8 pagesMaturity Onset Diabetes of The Young: Review ArticleMartha VillacisNo ratings yet
- HHS Public Access: Highlighting Diabetes - The Epidemic ContinuesDocument14 pagesHHS Public Access: Highlighting Diabetes - The Epidemic ContinuesRidhaNo ratings yet
- 6 175739 DiabetesDocument1 page6 175739 DiabetesNancy OcaNo ratings yet
- Clinical Review 102 Type 2 Diabetes Mellitus: Update On Diagnosis, Pathophysiology, and TreatmentDocument7 pagesClinical Review 102 Type 2 Diabetes Mellitus: Update On Diagnosis, Pathophysiology, and TreatmentWyn AgustinNo ratings yet
- TEMA 11 seminarioDM2adultosDocument17 pagesTEMA 11 seminarioDM2adultosJoyce LopezNo ratings yet
- 1 Octeto de DefronzoDocument23 pages1 Octeto de DefronzoLesli Rodriguez50% (2)
- E003290 FullDocument9 pagesE003290 FullANGELA CAMILA JARA LUZURIAGANo ratings yet
- Transcriptomic and Proteomic Analysis of Potential Therapeutic Target Genes in The Liver of Metformin Treated Sprague Dawley Rats With Type 2 Diabetes Mellitusijmm-41!06!3327Document15 pagesTranscriptomic and Proteomic Analysis of Potential Therapeutic Target Genes in The Liver of Metformin Treated Sprague Dawley Rats With Type 2 Diabetes Mellitusijmm-41!06!3327ErickNo ratings yet
- The Pancreas in Health and in Diabetes: Diabetologia (2020) 63:1962-1965Document4 pagesThe Pancreas in Health and in Diabetes: Diabetologia (2020) 63:1962-1965Juan Mendia OssioNo ratings yet
- The Protective Role of Smad7 in Diabetic Kidney Disease: Mechanism and Therapeutic PotentialDocument12 pagesThe Protective Role of Smad7 in Diabetic Kidney Disease: Mechanism and Therapeutic PotentialTam LyNo ratings yet
- Naskah PublikasiDocument11 pagesNaskah PublikasiKhairiyatul YasminNo ratings yet
- Cellular Mechanisms of Insulin Resistance.Document6 pagesCellular Mechanisms of Insulin Resistance.João AndradeNo ratings yet
- Chapter 6 by DR Anil KumarDocument9 pagesChapter 6 by DR Anil Kumarsanskargaglani03No ratings yet
- New insights into β-cell failure, regeneration and replacementDocument2 pagesNew insights into β-cell failure, regeneration and replacementYo MeNo ratings yet
- Kalra 2011Document7 pagesKalra 2011jinsung parkNo ratings yet
- Hdac Inhibition As A Novel Treatment For Diabetes MellitusDocument14 pagesHdac Inhibition As A Novel Treatment For Diabetes MellitusgreatmaleksNo ratings yet
- Correos Electrónicos Active Vitamin D3 Protects Against Diabetic Kidney Disease by Regulating The JNK Signaling Pathway in RatsDocument9 pagesCorreos Electrónicos Active Vitamin D3 Protects Against Diabetic Kidney Disease by Regulating The JNK Signaling Pathway in Ratsnoranton7No ratings yet
- Disruption of The 5-Lipoxygenase Pathway Attenuates Atherogenesis Consequent To COX-2 Deletion in MiceDocument6 pagesDisruption of The 5-Lipoxygenase Pathway Attenuates Atherogenesis Consequent To COX-2 Deletion in MicePaul KelnerNo ratings yet
- 979 7003 2 PBDocument8 pages979 7003 2 PBJesús Torres MayaNo ratings yet
- S146532492100030XDocument11 pagesS146532492100030XtiheloNo ratings yet
- Humans With Type 2 Diabetes: - Cell Deficit and Increased - Cell Apoptosis inDocument9 pagesHumans With Type 2 Diabetes: - Cell Deficit and Increased - Cell Apoptosis ingrilo604No ratings yet
- Maturity-Onset Diabetes of The Young Type 5 A MULTISYSTEMIC Disease: A CASE Report of A Novel Mutation in The HNF1B Gene and Literature ReviewDocument8 pagesMaturity-Onset Diabetes of The Young Type 5 A MULTISYSTEMIC Disease: A CASE Report of A Novel Mutation in The HNF1B Gene and Literature ReviewLaura SofiaNo ratings yet
- From The Triumvirate To The Ominous Octet: A New Paradigm For The Treatment of Type 2 Diabetes MellitusDocument26 pagesFrom The Triumvirate To The Ominous Octet: A New Paradigm For The Treatment of Type 2 Diabetes MellituselftotalNo ratings yet
- Genetics of Gestational Diabetes Mellitus and Type 2 DiabetesDocument7 pagesGenetics of Gestational Diabetes Mellitus and Type 2 Diabetessalman672003No ratings yet
- Xue 2021Document11 pagesXue 2021Camilla RequiãoNo ratings yet
- Expression of Advanced Glycation End Products and TheirDocument11 pagesExpression of Advanced Glycation End Products and TheirTamam JauharNo ratings yet
- Hao 2012Document9 pagesHao 2012vickydivi09No ratings yet
- Experimental Biology and MedicineDocument11 pagesExperimental Biology and MedicineYunitasya GuspiraNo ratings yet
- The Cellular Fate of Glucose and Its Relevance In-1-16Document16 pagesThe Cellular Fate of Glucose and Its Relevance In-1-16Yariela Amador MendietaNo ratings yet
- Genetic of DiabetesDocument4 pagesGenetic of Diabetesanghely leonela chuchuca naguaNo ratings yet
- Example of A Research Paper On DiabetesDocument5 pagesExample of A Research Paper On Diabetesijsgpibkf100% (1)
- Noninvasive Magnetic Resonance Imaging of Microvascular Changes in Type 1 DiabetesDocument6 pagesNoninvasive Magnetic Resonance Imaging of Microvascular Changes in Type 1 DiabetesAndrés Esteban Quezada VenegasNo ratings yet
- Adv Exp Med Biol 2020 Apr8 Maranta F PDFDocument24 pagesAdv Exp Med Biol 2020 Apr8 Maranta F PDFFernando SousaNo ratings yet
- A Deeper Dive Into Lipid Alterations in CKDDocument2 pagesA Deeper Dive Into Lipid Alterations in CKDAli TalalNo ratings yet
- WNT Signaling, A Novel Pathway For Coronary Artery Disease and Metabolic SyndromeDocument5 pagesWNT Signaling, A Novel Pathway For Coronary Artery Disease and Metabolic SyndromenamachiNo ratings yet
- Early Urinary Markers of Diabetic Kidney Disease: A Nested Case-Control Study From The Diabetes Control and Complications Trial (DCCT)Document11 pagesEarly Urinary Markers of Diabetic Kidney Disease: A Nested Case-Control Study From The Diabetes Control and Complications Trial (DCCT)Felipe SangiovanniNo ratings yet
- Medical Complications of Type 2 DiabetesDocument422 pagesMedical Complications of Type 2 DiabetesMayracpp.16No ratings yet
- Diabetic Nephropathy 1Document8 pagesDiabetic Nephropathy 1Kity SarkarNo ratings yet
- Utsav2, Et AlDocument16 pagesUtsav2, Et Alnadin nNo ratings yet
- Latent Autoimmune Diabetes in Adults (LADA)Document5 pagesLatent Autoimmune Diabetes in Adults (LADA)Fina Ahmad FitrianaNo ratings yet
- Understanding Insulin in The Age of Precision Medicine and Big Data - Under-Explored Nature of GenomicsDocument38 pagesUnderstanding Insulin in The Age of Precision Medicine and Big Data - Under-Explored Nature of GenomicsGestne AureNo ratings yet
- Liquid Biopsy T2DMDocument16 pagesLiquid Biopsy T2DMIgor VasićNo ratings yet
- Nihms 1840957Document35 pagesNihms 1840957Melda AngleNo ratings yet
- Editorial: Complications of DiabetesDocument6 pagesEditorial: Complications of DiabetesYULISSA FLORES RONDONNo ratings yet
- The Defi Nition of Disability: What Is in A Name?: CommentDocument3 pagesThe Defi Nition of Disability: What Is in A Name?: CommentLUIS EDUARDO VASQUEZ ESPINOZANo ratings yet
- Ijms 21 01770Document21 pagesIjms 21 01770Chyntia MadonaNo ratings yet
- Nesca2013 Article IdentificationOfParticularGrou PDFDocument10 pagesNesca2013 Article IdentificationOfParticularGrou PDFBelen GaleraNo ratings yet
- Use of The Estimated Glucose Disposal Rate As A Measure of Insulin Resistance in An Urban Multiethnic Population With Type 1 DiabetesDocument6 pagesUse of The Estimated Glucose Disposal Rate As A Measure of Insulin Resistance in An Urban Multiethnic Population With Type 1 Diabetesjulio perezNo ratings yet
- Therapeutic Effects of Adipose Stem Cells From Diabetic Mice For The Treatment of Type 2 DiabetesDocument10 pagesTherapeutic Effects of Adipose Stem Cells From Diabetic Mice For The Treatment of Type 2 DiabetesamrilmaenbNo ratings yet
- Trerapi Insulin Siip 2Document10 pagesTrerapi Insulin Siip 2Indra YudaNo ratings yet
- Diabetes 2 e Higado Graso e InhibidorrsDocument15 pagesDiabetes 2 e Higado Graso e InhibidorrsNadia PNo ratings yet
- Expression of Monosaccharide Transporters in Intestine of Diabetic HumansDocument8 pagesExpression of Monosaccharide Transporters in Intestine of Diabetic HumansmiminNo ratings yet
- Case Study Formate MSNDocument2 pagesCase Study Formate MSNLijoNo ratings yet
- Vasopressors For ShockDocument21 pagesVasopressors For ShocknugrahaNo ratings yet
- Infection and Tumorigenesis of Biomaterials by Smit Prajapati-200280103028 and Yukta Dodia - 200280103052Document18 pagesInfection and Tumorigenesis of Biomaterials by Smit Prajapati-200280103028 and Yukta Dodia - 200280103052Yukta DodiaNo ratings yet
- N-Ophth Disorders in HIVDocument6 pagesN-Ophth Disorders in HIVdwongNo ratings yet
- Closing The Cancer Divide: A Blueprint For ActionDocument45 pagesClosing The Cancer Divide: A Blueprint For ActionPresentaciones_FKNo ratings yet
- Objectives For Critical Care RotationDocument9 pagesObjectives For Critical Care Rotationsteviestevie333No ratings yet
- Oxford GastroenterologyDocument673 pagesOxford Gastroenterologymymamym100% (1)
- Principles of Removable Denture Pros Tho Don Tics 2007-08 - KaddahDocument145 pagesPrinciples of Removable Denture Pros Tho Don Tics 2007-08 - KaddahJudy Abbott100% (1)
- COPD Vs RLDDocument64 pagesCOPD Vs RLDXine DeeNo ratings yet
- tmpD824 TMPDocument12 pagestmpD824 TMPFrontiersNo ratings yet
- APA - DSM5 - Level 2 Inattention Parent of Child Age 6 To 17 PDFDocument3 pagesAPA - DSM5 - Level 2 Inattention Parent of Child Age 6 To 17 PDFLiana Storm0% (1)
- MPC-CPN Basics of Toxicology-HDocument12 pagesMPC-CPN Basics of Toxicology-HNahid ParveenNo ratings yet
- 07 - NDP Diving Emergency Assistance Plan SampleDocument2 pages07 - NDP Diving Emergency Assistance Plan Sample12scribd123No ratings yet
- Nursing Care Plan Guide: Subjective DataDocument1 pageNursing Care Plan Guide: Subjective DataJan Oliver YaresNo ratings yet
- Chapter 21 - Nursing Care of The Family During The Postpartum PeriodDocument11 pagesChapter 21 - Nursing Care of The Family During The Postpartum PeriodJill Hill100% (3)
- Patients-RightsDocument1 pagePatients-Rightsadalacse2016No ratings yet
- A Guide To Clinical Case Study and Its PresentationDocument13 pagesA Guide To Clinical Case Study and Its PresentationVince Troy AquinoNo ratings yet
- Testicular CancerDocument24 pagesTesticular CancerJulianne LeeNo ratings yet
- Critical Care Nurse Skills ChecklistDocument4 pagesCritical Care Nurse Skills ChecklistMichael Silva0% (1)
- Amee Guide No 81 Part 1Document10 pagesAmee Guide No 81 Part 1CatharinaWidiartiniNo ratings yet
- Revitears Study 2Document16 pagesRevitears Study 2office.hospimedNo ratings yet
- BLS Aha - 2010 Refresher Course For Junior Clerks 2015Document28 pagesBLS Aha - 2010 Refresher Course For Junior Clerks 2015Kalpana ShahNo ratings yet
- Pcap - PathophysiologyDocument4 pagesPcap - PathophysiologyAyla Mar100% (1)
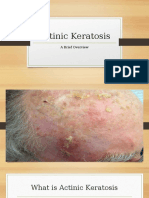
- Actinic KeratosisDocument19 pagesActinic KeratosisDajour CollinsNo ratings yet
- OB-Reviewer With AlgorithmDocument29 pagesOB-Reviewer With Algorithmriczen mae vilaNo ratings yet
- PamphletDocument2 pagesPamphletapi-25931970033% (3)
- CT and MRI of Abdomen and PelvicDocument1,691 pagesCT and MRI of Abdomen and PelvicĐức Khang NguyễnNo ratings yet
- NT To Look Better Than EVER This Summer? Why THIS Diet Plan Could Be The Answer (And You Can Still Eat Chocolate!)Document4 pagesNT To Look Better Than EVER This Summer? Why THIS Diet Plan Could Be The Answer (And You Can Still Eat Chocolate!)dreeNo ratings yet
- Marijuana and EpilepsyDocument17 pagesMarijuana and EpilepsyOmar AntabliNo ratings yet