Professional Documents
Culture Documents
Ionic Compound Naming Key
Uploaded by
nisrina amalia0 ratings0% found this document useful (0 votes)
16 views1 pageOriginal Title
Ionic-Compound-Naming-Key
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
16 views1 pageIonic Compound Naming Key
Uploaded by
nisrina amaliaCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
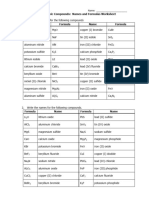
Name _______________________ Date ________________
IONIC COMPOUND NAMES AND FORMULAS
The name of an ionic compound indicates whether or not the
metal has a variable charge and whether the anion is
monatomic or polyatomic. Write the names and formulas of
these ionic compounds.
Name Formula
1 ZnCl2 zinc chloride 11 sodium hydroxide NaOH
2 TiI4 titanium(IV) iodide 12 copper(I) arsenide Cu3As
3 Ag2SO3 silver sulfite 13 beryllium chloride BeCl2
4 CaSO4 calcium sulfate 14 calcium oxide CaO
magnesium
5 Mg3P2 15 ammonium sulfite (NH4)2SO3
phosphide
6 K2CO3 potassium carbonate 16 iron(III) oxide Fe2O3
7 AlPO4 aluminum phosphate 17 vanadium(V) phosphate V3(PO4)5
ammonium
8 NH4OH 18 magnesium nitrate Mg(NO3)2
hydroxide
9 Co3N2 cobalt(II) nitride 19 silver cyanide AgCN
10 MgBr2 magnesium bromide 20 calcium phosphate Ca3(PO4)2
sciencenotes.org
You might also like
- IB Chemistry-Revision On Naming Ionic CompoundsDocument3 pagesIB Chemistry-Revision On Naming Ionic CompoundsRania ShabanNo ratings yet
- Chemical Formula Writing Worksheet SolutionsDocument3 pagesChemical Formula Writing Worksheet SolutionsReid Pineda Creencia100% (1)
- Ionic Compound Formula Writing Worksheet SolutionsDocument5 pagesIonic Compound Formula Writing Worksheet SolutionslalNo ratings yet
- Nomenclature WorksheetDocument5 pagesNomenclature WorksheetJapphetNo ratings yet
- Ionic Compound Names and FormulasDocument1 pageIonic Compound Names and FormulasTolga TunaboyluNo ratings yet
- WKS001 010 636149 PDFDocument2 pagesWKS001 010 636149 PDFjulsNo ratings yet
- Common Ions and Their ChargesDocument2 pagesCommon Ions and Their ChargesDip MajumderNo ratings yet
- Formulas and Names of Ionic CompoundsDocument3 pagesFormulas and Names of Ionic CompoundsMandanas GabrielNo ratings yet
- 2.4.3 Chemical Formula and Naming Practice QuestionsDocument7 pages2.4.3 Chemical Formula and Naming Practice Questionsphat.vuongNo ratings yet
- Naming - Compounds - Mixed With AnswersDocument4 pagesNaming - Compounds - Mixed With AnswerslinaNo ratings yet
- Answers Nomencalture Extra Practice PDFDocument3 pagesAnswers Nomencalture Extra Practice PDFAngel Joy CatalanNo ratings yet
- Here are the answers to the Counting Atoms worksheet:1) CaF22) Be(OH)2 3) NO24) Al2(SO4)35) NH4NO36) S2F2 7) Na2CO38) CH49) PCl310) Mg(OH)211) K3PO4 12) P2Br413) NH3Document14 pagesHere are the answers to the Counting Atoms worksheet:1) CaF22) Be(OH)2 3) NO24) Al2(SO4)35) NH4NO36) S2F2 7) Na2CO38) CH49) PCl310) Mg(OH)211) K3PO4 12) P2Br413) NH3Supremo DelagerNo ratings yet
- Chemical Nomenclature - Google DocsDocument7 pagesChemical Nomenclature - Google DocsQuỳnh NgânNo ratings yet
- Chemical Formula Writing WorksheetDocument5 pagesChemical Formula Writing WorksheetÂziz ShuvoNo ratings yet
- Naming CompoundsDocument3 pagesNaming Compoundsrobenroben155No ratings yet
- Review 1 WorksheetDocument2 pagesReview 1 WorksheetsupremeNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice Worksheet김동주No ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetOlivia DitzelNo ratings yet
- Ionic Compounds WorksheetDocument2 pagesIonic Compounds WorksheetBrillantes JYNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetSunshine LadyNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice Worksheetarlene serdiniaNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetBrillantes JYNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetSam JoNo ratings yet
- Baso Agno H S Cao H Co MG (Po) K Cro NaiDocument2 pagesBaso Agno H S Cao H Co MG (Po) K Cro NaiAmy BalicagNo ratings yet
- Even More Naming Ionic Compounds: This Work Is Licensed Under A and IsDocument2 pagesEven More Naming Ionic Compounds: This Work Is Licensed Under A and IsNeeta PandeyNo ratings yet
- Review #3 NomenclatureDocument1 pageReview #3 NomenclatureCassandra MachadoNo ratings yet
- Laboratory Periodic Table: Gsci1103L-General Chemistry 1 LabDocument6 pagesLaboratory Periodic Table: Gsci1103L-General Chemistry 1 LabAndrea AurielleNo ratings yet
- Microsoft Word - Ionic-CovalentNameRace1Document2 pagesMicrosoft Word - Ionic-CovalentNameRace1cen BsitNo ratings yet
- T1 - AtomicStructure and PTableSLOP AnswersDocument12 pagesT1 - AtomicStructure and PTableSLOP AnswersboobooNo ratings yet
- Simple Binary Ionic Compounds: Nomenclature Worksheet 2Document4 pagesSimple Binary Ionic Compounds: Nomenclature Worksheet 2NameNo ratings yet
- 5-Ternary Ionic CompoundsDocument1 page5-Ternary Ionic CompoundsmargaritaisabellechamNo ratings yet
- More Nomenclature PracticeDocument2 pagesMore Nomenclature PracticeeapicciottoNo ratings yet
- Answers - Naming Chemical CompoundsDocument3 pagesAnswers - Naming Chemical CompoundsIvy JoyceNo ratings yet
- Unit 04 - Study Guide - ANSWERSDocument2 pagesUnit 04 - Study Guide - ANSWERSBipin GhimireNo ratings yet
- Inorganic Compounds: Chemical Name Chemical FormulaDocument6 pagesInorganic Compounds: Chemical Name Chemical FormulaFrendick LegaspiNo ratings yet
- Chemical Formula Writing Exercise - Cations and AnionsDocument5 pagesChemical Formula Writing Exercise - Cations and AnionsQusai Saify100% (3)
- Compound Names and FormulasDocument0 pagesCompound Names and FormulasMax SaubermanNo ratings yet
- Honors Chemistry WKSHT Names and Formulas V and ANSWERSDocument2 pagesHonors Chemistry WKSHT Names and Formulas V and ANSWERSkijijisellerNo ratings yet
- Naming Inorganic Compounds WorksheetDocument8 pagesNaming Inorganic Compounds Worksheettalktotiffanycheng100% (1)
- 5.9 Polyatomic CompoundsDocument3 pages5.9 Polyatomic Compoundsmichael.delaney8541No ratings yet
- Naming Ionic Compounds Practice Worksheet - SolutionsDocument3 pagesNaming Ionic Compounds Practice Worksheet - SolutionsJa Son Tonogbanua100% (1)
- SCH3U0 Nomenclature PracticeDocument7 pagesSCH3U0 Nomenclature PracticeArmann JohalNo ratings yet
- Chapter - 7 Correction Naming CompoundsDocument2 pagesChapter - 7 Correction Naming CompoundsMurad IsayevNo ratings yet
- Ternary CompoundsDocument27 pagesTernary CompoundsIam PaulNo ratings yet
- 50 Geology Minerals: Alphabetically WrittenDocument2 pages50 Geology Minerals: Alphabetically Writtenorjiugo.victorNo ratings yet
- Activity5 ChemicalformulasDocument2 pagesActivity5 ChemicalformulasJohn Hayden Dela CruzNo ratings yet
- Chem (LAS)Document2 pagesChem (LAS)mhyrela roncedNo ratings yet
- Cation Positive Ion Charge ChartDocument6 pagesCation Positive Ion Charge ChartSEAW FUI MINGNo ratings yet
- Post 5.9. Ionic Compounds Practice - ANSWERSDocument3 pagesPost 5.9. Ionic Compounds Practice - ANSWERSAlan MartínNo ratings yet
- Naming Review Practice Answer KeyDocument1 pageNaming Review Practice Answer Keyapi-376281962No ratings yet
- 3 Ionic Compounds Assign - AnswersDocument1 page3 Ionic Compounds Assign - Answersapi-272986951No ratings yet
- Naming Chemical Compounds WorksheetDocument4 pagesNaming Chemical Compounds WorksheetChii YenNo ratings yet
- Marvin M. Pagli-WPS OfficeDocument4 pagesMarvin M. Pagli-WPS OfficeJohn Kenneth CoritanaNo ratings yet
- Pcqa111 - Assignment For Nomenclature and Formula WritingDocument1 pagePcqa111 - Assignment For Nomenclature and Formula WritingRusselle Kate AlvaradoNo ratings yet
- UNIT 7 Review AnswersDocument3 pagesUNIT 7 Review AnswersmamazookeeprNo ratings yet
- Name and write formulas for common compoundsDocument1 pageName and write formulas for common compoundsMarchelle MondezNo ratings yet
- Ionic Compounds Names and Formulas Worksheet AnswersDocument2 pagesIonic Compounds Names and Formulas Worksheet AnswersShayan UzzamanNo ratings yet
- Coordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972From EverandCoordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972A. B. P. LeverNo ratings yet
- Iron Metabolism: From Molecular Mechanisms to Clinical ConsequencesFrom EverandIron Metabolism: From Molecular Mechanisms to Clinical ConsequencesRating: 5 out of 5 stars5/5 (1)