Professional Documents
Culture Documents
Naming Review Practice Answer Key
Uploaded by
api-3762819620 ratings0% found this document useful (0 votes)
29 views1 pageOriginal Title
naming review practice answer key
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
29 views1 pageNaming Review Practice Answer Key
Uploaded by
api-376281962Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
Naming Review Practice
Directions: On the left of each question, identify if the compound/molecule is Ionic (write
an I) or Covalent (write a C). Then, either write the chemical name of the molecule (if
given the formula) or the chemical formula of the molecule (if given the name)
Covalent 1. CS3 Carbon Trisulfide
Ionic 2. TiO2 Titanium IV Oxide
Ionic 3. CaCO3 Calcium Carbonate
Ionic 4. Cu(NO2)2 Copper II Nitrite
Ionic 5. Sn(CN)4 Tin IV Cyanide
Ionic 6. Ag2SO3 Silver Sulfite
Ionic 7. NiSe2 Nickel IV Selenide
Covalent 8. P3O5 Triphosphorus Pentoxide
Ionic 9. Sn3(PO4)2
Tin II Phosphate
Covalent 10. N7Cl3 Heptanitrogen Trichloride
Ionic 11. Manganese (III) bromide MnBr3
Covalent 12. Sulfur dioxide
SO2
Ionic 13. Tin (IV) sulfate
Sn(SO4)2
Ionic 14. Zinc hydroxide Zn(OH)2
Covalent 15. Carbon tetrachloride CCl4
Ionic 16. Barium cyanide
Ba(CN)2
Ionic 17. Cobalt (VI) Chromate
Co(CrO4)3
Ionic 18. Lithium nitrite LiNO2
Covalent 19. Trisulfur hexafluoride S3F6
Ionic 20. Aluminum oxalate
Al2(C2O4)3
You might also like
- Analytical Chemistry of Niobium and Tantalum: International Series of Monographs on Analytical ChemistryFrom EverandAnalytical Chemistry of Niobium and Tantalum: International Series of Monographs on Analytical ChemistryRating: 3.5 out of 5 stars3.5/5 (3)
- Naming Review PracticeDocument1 pageNaming Review Practiceapi-376281962No ratings yet
- Coordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972From EverandCoordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972A. B. P. LeverNo ratings yet
- Naming Review PracticeDocument1 pageNaming Review Practiceapi-376281962No ratings yet
- Handbook of Preparative Inorganic Chemistry V2From EverandHandbook of Preparative Inorganic Chemistry V2Georg BrauerNo ratings yet
- Nomenclature Review AssignmentDocument8 pagesNomenclature Review AssignmentTish BarnesNo ratings yet
- Simple Binary Ionic Compounds: Nomenclature Worksheet 2Document4 pagesSimple Binary Ionic Compounds: Nomenclature Worksheet 2NameNo ratings yet
- Laboratory Periodic Table: Gsci1103L-General Chemistry 1 LabDocument6 pagesLaboratory Periodic Table: Gsci1103L-General Chemistry 1 LabAndrea AurielleNo ratings yet
- UNIT 7 Review AnswersDocument3 pagesUNIT 7 Review AnswersmamazookeeprNo ratings yet
- Pcqa111 - Assignment For Nomenclature and Formula WritingDocument1 pagePcqa111 - Assignment For Nomenclature and Formula WritingRusselle Kate AlvaradoNo ratings yet
- Ionic Nomenclature PracticeDocument5 pagesIonic Nomenclature PracticevanammanNo ratings yet
- 5-Ternary Ionic CompoundsDocument1 page5-Ternary Ionic CompoundsmargaritaisabellechamNo ratings yet
- 5.7-5.10 Naming Mixed Ionic and Covalent Compounds AnswersDocument2 pages5.7-5.10 Naming Mixed Ionic and Covalent Compounds AnswersAlan MartínNo ratings yet
- Answers Nomencalture Extra Practice PDFDocument3 pagesAnswers Nomencalture Extra Practice PDFAngel Joy CatalanNo ratings yet
- Inorganic Nomenclature Rules, Examples and Practice Problems Bansal PatternDocument7 pagesInorganic Nomenclature Rules, Examples and Practice Problems Bansal PatternKumarNo ratings yet
- Honors Chemistry WKSHT Names and Formulas V and ANSWERSDocument2 pagesHonors Chemistry WKSHT Names and Formulas V and ANSWERSkijijisellerNo ratings yet
- Chem (LAS)Document2 pagesChem (LAS)mhyrela roncedNo ratings yet
- Chemistry - WS Combo #1Document2 pagesChemistry - WS Combo #1thomasnakyra623No ratings yet
- Namingpacketanswers 3Document14 pagesNamingpacketanswers 3Supremo DelagerNo ratings yet
- Naming - Compounds - Mixed With AnswersDocument4 pagesNaming - Compounds - Mixed With AnswerslinaNo ratings yet
- Microsoft Word - Ionic-CovalentNameRace1Document2 pagesMicrosoft Word - Ionic-CovalentNameRace1cen BsitNo ratings yet
- Q1Document1 pageQ1Jant Erbert GarbosoNo ratings yet
- Ionic Compound Naming KeyDocument1 pageIonic Compound Naming Keynisrina amaliaNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice Worksheet김동주No ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice Worksheetarlene serdiniaNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetBrillantes JYNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetSunshine LadyNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetOlivia DitzelNo ratings yet
- Review 1 WorksheetDocument2 pagesReview 1 WorksheetsupremeNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetSam JoNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetBrillantes JYNo ratings yet
- IB Chemistry-Revision On Naming Ionic CompoundsDocument3 pagesIB Chemistry-Revision On Naming Ionic CompoundsRania ShabanNo ratings yet
- Dustin Kern - Writing Polyatomic Names and Formulas WorksheetDocument2 pagesDustin Kern - Writing Polyatomic Names and Formulas Worksheetapi-644456294No ratings yet
- Chapter - 7 Correction Naming CompoundsDocument2 pagesChapter - 7 Correction Naming CompoundsMurad IsayevNo ratings yet
- Naming Ionic Compounds Practice Worksheet - SolutionsDocument3 pagesNaming Ionic Compounds Practice Worksheet - SolutionsJa Son Tonogbanua100% (1)
- Jordan Paddock - Writing Polyatomic Names and Formulas WorksheetDocument1 pageJordan Paddock - Writing Polyatomic Names and Formulas Worksheetapi-675312232No ratings yet
- Nomenclature AnsDocument8 pagesNomenclature Ansdlc352-sc1No ratings yet
- WKS001 010 636149 PDFDocument2 pagesWKS001 010 636149 PDFjulsNo ratings yet
- Formula Writing - CambridgeDocument5 pagesFormula Writing - CambridgeQusai Saify100% (3)
- Lab 03 Chemical NomenclatureDocument2 pagesLab 03 Chemical Nomenclaturewidowspider100% (1)
- Max Hyman - Writing Mixed Names and Formulas WorksheetDocument1 pageMax Hyman - Writing Mixed Names and Formulas Worksheetapi-673504994No ratings yet
- Unit 04 - Study Guide - ANSWERSDocument2 pagesUnit 04 - Study Guide - ANSWERSBipin GhimireNo ratings yet
- Lesson 1.0 - Questions 1. Write Standard Atomic Notation For H Ca F Li ODocument16 pagesLesson 1.0 - Questions 1. Write Standard Atomic Notation For H Ca F Li OThai NgoNo ratings yet
- WS#2 Naming and Writing Inorganic CompoundsDocument6 pagesWS#2 Naming and Writing Inorganic CompoundsOw ZeeNo ratings yet
- Baso Agno H S Cao H Co MG (Po) K Cro NaiDocument2 pagesBaso Agno H S Cao H Co MG (Po) K Cro NaiAmy BalicagNo ratings yet
- SCH3U0 Nomenclature PracticeDocument7 pagesSCH3U0 Nomenclature PracticeArmann JohalNo ratings yet
- Valencies NewDocument4 pagesValencies NewPrathamesh ParmarNo ratings yet
- Inorganic Compounds: Chemical Name Chemical FormulaDocument6 pagesInorganic Compounds: Chemical Name Chemical FormulaFrendick LegaspiNo ratings yet
- ch10 Nomenclature ReportDocument3 pagesch10 Nomenclature Reportapi-233552637No ratings yet
- Chemical Formula Writing Worksheet SolutionsDocument3 pagesChemical Formula Writing Worksheet SolutionsReid Pineda Creencia100% (1)
- Common Cations, Anions, Acids, Salts.Document2 pagesCommon Cations, Anions, Acids, Salts.Jas MeeraNo ratings yet
- Cation and AnionDocument1 pageCation and Anionsumityadav742008No ratings yet
- Review #3 NomenclatureDocument1 pageReview #3 NomenclatureCassandra MachadoNo ratings yet
- Marvin M. Pagli-WPS OfficeDocument4 pagesMarvin M. Pagli-WPS OfficeJohn Kenneth CoritanaNo ratings yet
- Answers of Exercise 1 (A)Document6 pagesAnswers of Exercise 1 (A)Lisa SinhaNo ratings yet
- Ternary CompoundsDocument27 pagesTernary CompoundsIam PaulNo ratings yet
- Ionic Compounds Worksheet-IiiDocument1 pageIonic Compounds Worksheet-IiitylerNo ratings yet
- Naming Multivalent Ionic Compounds WsDocument1 pageNaming Multivalent Ionic Compounds Wsapi-246864303No ratings yet
- Assessment Chapter 4 Group 2Document9 pagesAssessment Chapter 4 Group 2masya marchelinaNo ratings yet
- Unit 4 Naming Amp Types Naming Polyatomic Compounds Worksheet 2 Page 1 AnswersDocument2 pagesUnit 4 Naming Amp Types Naming Polyatomic Compounds Worksheet 2 Page 1 AnswersdiahemaNo ratings yet
- Limiting Reactants Notes HandoutDocument9 pagesLimiting Reactants Notes Handoutapi-376281962No ratings yet
- Interactive Simulation RubricDocument1 pageInteractive Simulation Rubricapi-376281962No ratings yet
- Interactive Simulation RubricDocument1 pageInteractive Simulation Rubricapi-376281962No ratings yet
- Gummy Bear InstructionsDocument1 pageGummy Bear Instructionsapi-376281962No ratings yet
- Gummy Bear InstructionsDocument1 pageGummy Bear Instructionsapi-376281962No ratings yet
- Stoichiometry Notes HandoutDocument9 pagesStoichiometry Notes Handoutapi-376281962No ratings yet
- Gummi Bear Poster RubricDocument2 pagesGummi Bear Poster Rubricapi-376281962No ratings yet
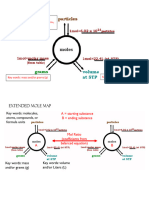
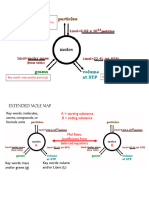
- Mole Map Handout UploadDocument1 pageMole Map Handout Uploadapi-376281962No ratings yet
- Dimensional Analysis Extra Practice Answer KeyDocument3 pagesDimensional Analysis Extra Practice Answer Keyapi-376281962No ratings yet
- Gummi Bear Poster RubricDocument2 pagesGummi Bear Poster Rubricapi-376281962No ratings yet
- Stoichiometry Practice Answer KeyDocument1 pageStoichiometry Practice Answer Keyapi-376281962No ratings yet
- Edpuzzle Lecture RubricDocument1 pageEdpuzzle Lecture Rubricapi-376281962No ratings yet
- Limiting Reactants Extra PracticeDocument2 pagesLimiting Reactants Extra Practiceapi-376281962No ratings yet
- Limiting Reactants Extra Practice Answer KeyDocument2 pagesLimiting Reactants Extra Practice Answer Keyapi-376281962No ratings yet
- Mole Map Handout UploadDocument1 pageMole Map Handout Uploadapi-376281962No ratings yet
- Assessment RubricDocument1 pageAssessment Rubricapi-376281962No ratings yet
- Edpuzzle Lecture RubricDocument1 pageEdpuzzle Lecture Rubricapi-376281962No ratings yet
- Assessment RubricDocument1 pageAssessment Rubricapi-376281962No ratings yet
- Assessment RubricDocument1 pageAssessment Rubricapi-376281962No ratings yet
- Mole Conversions Practice Answer KeyDocument1 pageMole Conversions Practice Answer Keyapi-376281962No ratings yet
- Edpuzzle Lecture RubricDocument1 pageEdpuzzle Lecture Rubricapi-376281962No ratings yet
- Assessment RubricDocument1 pageAssessment Rubricapi-376281962No ratings yet
- Dimensional Analysis Extra PracticeDocument1 pageDimensional Analysis Extra Practiceapi-376281962No ratings yet
- SyllabusDocument2 pagesSyllabusapi-376281962No ratings yet
- Lesson Plan 3Document8 pagesLesson Plan 3api-376281962No ratings yet
- Discussion RubricDocument1 pageDiscussion Rubricapi-376281962No ratings yet
- Discussion RubricDocument1 pageDiscussion Rubricapi-376281962No ratings yet
- Discussion RubricDocument1 pageDiscussion Rubricapi-376281962No ratings yet
- Lesson Plan 4Document8 pagesLesson Plan 4api-376281962No ratings yet