Professional Documents
Culture Documents
Stoichiometry Practice Answer Key
Uploaded by
api-376281962Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Stoichiometry Practice Answer Key
Uploaded by
api-376281962Copyright:
Available Formats
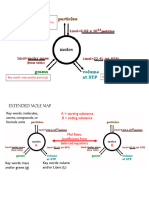
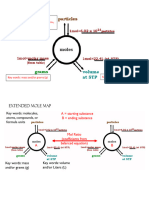
The following conversions can be from 1 step to 3 steps and may or may not contain mol ratios.
Always map
out your steps using your mol map to figure out what needs to be used!
1. Balance the following equation then solve the following problems
__N2 + _3_H2 ➔ 2._N H3
a. How many moles of hydrogen gas are needed to produce 2 moles of ammonia?
b. How many moles of hydrogen gas are needed to produce 6 moles of ammonia?
c. How many moles of hydrogen gas are needed to produce 136 grams of ammonia?
i3<odNl� -l lrv-ol/vH.;.
N=fx/L/.OD7=1'-I.OV7 ;,< >< 312?nlH, =-
17- 02, I
1-\ : 3 ;,r. 1. OD 8 =- 3. Q;>LI zJ j\} H_) .,,7�1 ,'\Jr+
__,
J7.o.3l o/Y>'-0 1 ➔ l�I NH., =- !7.031
Nt-t3
3
d. How many molecules of nitrogen gas are needed to produce 12 molecules of ammonia?
I w--ol tJ \--13 3
/:) � c.. N+I� ;,( ,.o) J</0� )'Y'O�C NJ..
'7.0J xrl 3 t')"O�e, tJH� I vv-ol tJ ;i---
e. How many grams of nitrogen gas are needed to produce 119 grams of ammonia?
N
-t fu\/11 .tJ C, } � I N i-/ 3 =- J7. 013 d N H _, i"
I I q d N H3x l \V'O l N \-t -3 x
\ YNJ I N� ?-8 • 0 I L.} fJ-J.
N :J� J/../.Ol>7-= ;28.0IY 17.013 NH.") )v-<> IN� 3 >< )rNJ)
_ry>--c)t 1\Jd
:J
;; I »-o \ N :>- =- Jg_ 0 H .1 Nd- �; 9 7. en 4' j\) � l
f. Calculate the mass of ammonia that could be produces with 55.8 L of hydrogen gas.
-ft:f'-vvn,. tr c., 55.8 L H \vi,-ol �Jo :?Yv"O/
>< -- -- NH
->< 17.013 cJ N H3,
I Vval NH.:, ::: /7.0l1 Nl-l:!> *
�x -3
0 ;2.:;,.y L � 3�) H J. I w-ol NH ,)
).
J8'.�5L-J
g. How many grams of hydrogen gas are needed to produce 8.57 x 10 molecules bf'amrnonia1
34
f- 4 J\lrl3 /
=--
H : ) 'I- I. lTD F = .2. 0 I (,;, YM) /
� I vY'--0 I �.;z -== .2 • O I 0 � 1-J-�
'6.S7 i-JD '1 mol.ec Ni-1
3
x \l'h-01 N�� ? ol'1 fl H�
3 X X
b. OJ. -,I. ID� 3 Y'>"-0 [; c. N� ;)�/ NH :> lr-i---ol H,)_
1 3---_=H; \
l��-,0-=1;_-
You might also like
- Nitrogen Cycle ppt-1Document14 pagesNitrogen Cycle ppt-1api-293001217No ratings yet
- 55 bài đọc N5Document109 pages55 bài đọc N5Trang Ngân100% (2)
- Piping Design Articles by Robert Kern PDFDocument53 pagesPiping Design Articles by Robert Kern PDFDiego IQ100% (1)
- Solution Manual for The Elements of Polymer Science and EngineeringFrom EverandSolution Manual for The Elements of Polymer Science and EngineeringRating: 4 out of 5 stars4/5 (3)
- VTU Exam Question Paper With Solution of 18ME61 Finite Element Methods Aug-2022-Prashant S. HattiDocument53 pagesVTU Exam Question Paper With Solution of 18ME61 Finite Element Methods Aug-2022-Prashant S. HattiFusion gaming100% (1)
- Mole Conversions Practice Answer KeyDocument1 pageMole Conversions Practice Answer Keyapi-376281962No ratings yet
- Mole Conversions Extra Practice Answer KeyDocument1 pageMole Conversions Extra Practice Answer Keyapi-376281962No ratings yet
- Esi - Ji Hecct: Ben - T 1 atDocument5 pagesEsi - Ji Hecct: Ben - T 1 atyaseen sheikhNo ratings yet
- E1 PDFDocument2 pagesE1 PDFAARSH GANDHINo ratings yet
- Lusiana Nurhayati Siregar Tugas 2 Bandar UdaraDocument5 pagesLusiana Nurhayati Siregar Tugas 2 Bandar UdaraLusiana Nurhayati SiregarNo ratings yet
- Pset10 Soln PDFDocument6 pagesPset10 Soln PDFBabasrinivas GuduruNo ratings yet
- Kinetics Refresher WS Answer KeyDocument2 pagesKinetics Refresher WS Answer KeyIchwan Permana MadaniNo ratings yet
- TTJ/JV-: Result Sheet - Experiment No. 2 (Characteristic of Venturi Meter)Document7 pagesTTJ/JV-: Result Sheet - Experiment No. 2 (Characteristic of Venturi Meter)Muhd AdamNo ratings yet
- Electric Charge and FieldDocument22 pagesElectric Charge and FieldJyotsanaNo ratings yet
- Mass Transfer - Chapter 2Document4 pagesMass Transfer - Chapter 2Man ChungNo ratings yet
- Base Number System ActivityDocument4 pagesBase Number System ActivityGwyneth NasingNo ratings yet
- Punjab GeographyDocument2 pagesPunjab Geographys90615100No ratings yet
- HE Natural ConvectionDocument10 pagesHE Natural ConvectionRinchiNo ratings yet
- F Dwed Fli R: LK Ol of ofDocument4 pagesF Dwed Fli R: LK Ol of ofLynn ShiyayoNo ratings yet
- Exercicis Serie 1Document3 pagesExercicis Serie 1rllach34No ratings yet
- Q) / Y+:: LB: VVTF LDocument7 pagesQ) / Y+:: LB: VVTF LCris FernandezNo ratings yet
- 21BCM0033 VL2021220507033 Ast01Document8 pages21BCM0033 VL2021220507033 Ast01Pawan PatroNo ratings yet
- Statistics AssignmentDocument15 pagesStatistics AssignmentAnmol YadavNo ratings yet
- Kishenmurali DF170184 BFC21303 Q1Document4 pagesKishenmurali DF170184 BFC21303 Q1kishen972No ratings yet
- Red! KJ (1: Fy RJDocument4 pagesRed! KJ (1: Fy RJMuhammad Rizwan QureshiNo ratings yet
- Experiment No. 4 Energy Gap: TejjjpDocument4 pagesExperiment No. 4 Energy Gap: Tejjjpname nameNo ratings yet
- Ilovepdf MergedDocument4 pagesIlovepdf MergedJose joel suncionNo ratings yet
- Finalexam SolutionsDocument8 pagesFinalexam SolutionsJonah BallNo ratings yet
- Diffraction Grating ObsDocument5 pagesDiffraction Grating ObsdudeNo ratings yet
- ZN Ni CR: Eng. I Treatrnent AnbariDocument13 pagesZN Ni CR: Eng. I Treatrnent AnbariRahul GuptaNo ratings yet
- Ios FLciV1Hd3JTF3MScDocument14 pagesIos FLciV1Hd3JTF3MScmknvjx9g9qNo ratings yet
- S0 :Su.,R Or.. - U.R..Rjc.: F'./1'.:Wpkel:1 Maa.L MTSTTKDocument5 pagesS0 :Su.,R Or.. - U.R..Rjc.: F'./1'.:Wpkel:1 Maa.L MTSTTKNavpreet DhimanNo ratings yet
- موضوعين في الفيزياء 2 ثانويDocument4 pagesموضوعين في الفيزياء 2 ثانويAmir MirouNo ratings yet
- It Ex1vesseul: E (Lec - Tiveness ''Document6 pagesIt Ex1vesseul: E (Lec - Tiveness ''حارث عامر محمدNo ratings yet
- Scan - Parcial LXDocument5 pagesScan - Parcial LXGabriela NiñoNo ratings yet
- Eiiil: VL' (Q.T)Document40 pagesEiiil: VL' (Q.T)Ahmed JamalNo ratings yet
- A06 Da Ise1.2Document5 pagesA06 Da Ise1.2Sanket KembalkarNo ratings yet
- NF04 - TD 2 - Exo3-4Document4 pagesNF04 - TD 2 - Exo3-4JohnsonNo ratings yet
- MPN 0188037 WDocument7 pagesMPN 0188037 WPatrickNo ratings yet
- Upload 1Document6 pagesUpload 1Cris FernandezNo ratings yet
- TTL'S.: .P E.R V::lg'f-O - , (IQ-6"Document5 pagesTTL'S.: .P E.R V::lg'f-O - , (IQ-6"Hei Kan ChengNo ratings yet
- hw6t OptimizationDocument4 pageshw6t Optimizationsamchen984No ratings yet
- Exam1 2009 KeyDocument7 pagesExam1 2009 KeyMark CarpesoNo ratings yet
- Maths FST ExamDocument5 pagesMaths FST ExambibinNo ratings yet
- Atomic Structure 4th March 2024Document12 pagesAtomic Structure 4th March 2024shishiranand25No ratings yet
- ResultsDocument2 pagesResultsMohamed Samir MohamedNo ratings yet
- Adobe Scan 12 Mar 2021Document10 pagesAdobe Scan 12 Mar 2021Ameya KotraNo ratings yet
- Math KZDocument6 pagesMath KZaibekzhanat7No ratings yet
- Test1 1Document4 pagesTest1 1api-3807258No ratings yet
- C2Document12 pagesC2dfdfNo ratings yet
- 0-1 Sets Method - Knapsack Problem - CO-3Document2 pages0-1 Sets Method - Knapsack Problem - CO-3Sasidhar chowdaryNo ratings yet
- Complex Numbers and Trig AssessmentDocument6 pagesComplex Numbers and Trig AssessmentAmael AN84788No ratings yet
- Sol UCP2 Mid v2Document3 pagesSol UCP2 Mid v2SandhiNo ratings yet
- Limiting Reactants Extra Practice Answer KeyDocument2 pagesLimiting Reactants Extra Practice Answer Keyapi-376281962No ratings yet
- Kfywo: d!9PL-ryDocument5 pagesKfywo: d!9PL-ryRudransh RatanNo ratings yet
- Apes Math DiagnosticDocument6 pagesApes Math Diagnosticdhbq665No ratings yet
- Units and DimensionsDocument4 pagesUnits and DimensionsRudrakshi SamalNo ratings yet
- Ejercicios EquilibrioDocument8 pagesEjercicios EquilibrioMariana Lopez MurrietaNo ratings yet
- Trigonometry Class Test: 18/7/11 ACE-Learning Worksheet © ACE-Learning Systems Pte LTDDocument5 pagesTrigonometry Class Test: 18/7/11 ACE-Learning Worksheet © ACE-Learning Systems Pte LTDvr72here3168No ratings yet
- EXP10 Long ReportDocument16 pagesEXP10 Long ReportDumishka MadhumalNo ratings yet
- Dividing Polynomials: Homework 6: Directions: UseDocument2 pagesDividing Polynomials: Homework 6: Directions: UsePaulina TorresNo ratings yet
- Ion Beams for Materials AnalysisFrom EverandIon Beams for Materials AnalysisR. Curtis BirdNo ratings yet
- Interactive Simulation RubricDocument1 pageInteractive Simulation Rubricapi-376281962No ratings yet
- Gummy Bear InstructionsDocument1 pageGummy Bear Instructionsapi-376281962No ratings yet
- Stoichiometry Notes HandoutDocument9 pagesStoichiometry Notes Handoutapi-376281962No ratings yet
- Interactive Simulation RubricDocument1 pageInteractive Simulation Rubricapi-376281962No ratings yet
- Limiting Reactants Notes HandoutDocument9 pagesLimiting Reactants Notes Handoutapi-376281962No ratings yet
- Gummi Bear Poster RubricDocument2 pagesGummi Bear Poster Rubricapi-376281962No ratings yet
- Edpuzzle Lecture RubricDocument1 pageEdpuzzle Lecture Rubricapi-376281962No ratings yet
- Mole Map Handout UploadDocument1 pageMole Map Handout Uploadapi-376281962No ratings yet
- Limiting Reactants Extra Practice Answer KeyDocument2 pagesLimiting Reactants Extra Practice Answer Keyapi-376281962No ratings yet
- Limiting Reactants Extra PracticeDocument2 pagesLimiting Reactants Extra Practiceapi-376281962No ratings yet
- Mole Map Handout UploadDocument1 pageMole Map Handout Uploadapi-376281962No ratings yet
- Periodic TableDocument1 pagePeriodic Tableapi-376281962No ratings yet
- Edpuzzle Lecture RubricDocument1 pageEdpuzzle Lecture Rubricapi-376281962No ratings yet
- Dimensional Analysis Extra PracticeDocument1 pageDimensional Analysis Extra Practiceapi-376281962No ratings yet
- Assessment RubricDocument1 pageAssessment Rubricapi-376281962No ratings yet
- Dimensional Analysis Extra Practice Answer KeyDocument3 pagesDimensional Analysis Extra Practice Answer Keyapi-376281962No ratings yet
- Edpuzzle Lecture RubricDocument1 pageEdpuzzle Lecture Rubricapi-376281962No ratings yet
- Discussion RubricDocument1 pageDiscussion Rubricapi-376281962No ratings yet
- SyllabusDocument2 pagesSyllabusapi-376281962No ratings yet
- Naming Review Practice Answer KeyDocument1 pageNaming Review Practice Answer Keyapi-376281962No ratings yet
- RESENSI Buku Juru Bicara TuhanDocument44 pagesRESENSI Buku Juru Bicara TuhanVirbyansah Achmadan NurrohmanNo ratings yet
- P Block Elements (Group 15, 16, 17 & 18) - JEE Main 2024 January Question Bank - MathonGoDocument6 pagesP Block Elements (Group 15, 16, 17 & 18) - JEE Main 2024 January Question Bank - MathonGorohansardar0102No ratings yet
- Astronaut and System Performance During Gemini EVADocument9 pagesAstronaut and System Performance During Gemini EVABob AndrepontNo ratings yet
- Cia-Zanzibar: The Hundred Days' RevolutionDocument170 pagesCia-Zanzibar: The Hundred Days' RevolutionMZALENDO.NET100% (1)
- Write The Following CompoundsDocument2 pagesWrite The Following Compoundsfabian1710No ratings yet
- Nitric Acid Manufacturing Flow SheetDocument2 pagesNitric Acid Manufacturing Flow Sheethana_gondokusumoNo ratings yet
- LulllDocument166 pagesLulllShoaib AliNo ratings yet
- Water LE 1 Surface TensionDocument2 pagesWater LE 1 Surface Tensionpradeep kumarNo ratings yet
- Oxygene ConcentratorDocument17 pagesOxygene ConcentratoradeNo ratings yet
- التحديات الفكرية المعاصرة وتجديد علم الكلامDocument43 pagesالتحديات الفكرية المعاصرة وتجديد علم الكلامcreation chamNo ratings yet
- Centrometal PelTec Kotao Na Pelete Tehnicke UputeDocument109 pagesCentrometal PelTec Kotao Na Pelete Tehnicke UputelagusterNo ratings yet
- HECATE-La Reina Del Infierno PDFDocument252 pagesHECATE-La Reina Del Infierno PDFJosephine Pinto GallardoNo ratings yet
- Biological Nitrogen FixationDocument7 pagesBiological Nitrogen FixationSushma KannapiranNo ratings yet
- Nurul Afina Shafie Year 10Sc1, 2011 Sayyidina Otman Secondary School, TutongDocument26 pagesNurul Afina Shafie Year 10Sc1, 2011 Sayyidina Otman Secondary School, TutongAfina ShsNo ratings yet
- Análisis Transaccional Alain Cardon Vncent Lenhardt Pierre Nicolas - Compressed-91-176Document86 pagesAnálisis Transaccional Alain Cardon Vncent Lenhardt Pierre Nicolas - Compressed-91-176jesus felixNo ratings yet
- H2S SeriesB12 - DatasheetDocument1 pageH2S SeriesB12 - DatasheetKareem RMGNo ratings yet
- Nitrogen CycleDocument9 pagesNitrogen CyclePearl BraganzaNo ratings yet
- Jurnal SkripsiDocument9 pagesJurnal SkripsiismihrpNo ratings yet
- Bab 10 - Animasi TB Penukargantian Metana - EnglishDocument36 pagesBab 10 - Animasi TB Penukargantian Metana - Englishrudi_zNo ratings yet
- Amin DKK 2016Document7 pagesAmin DKK 2016Athir Muhammad FakharNo ratings yet
- The Nitrogen CycleDocument14 pagesThe Nitrogen Cyclebarrybogs19No ratings yet
- Be Great - Ebook of 365 Inspirational QuotesDocument192 pagesBe Great - Ebook of 365 Inspirational Quotesnagesh2013No ratings yet
- BHP ALL UNIT NewDocument48 pagesBHP ALL UNIT NewDinda Nurafni HapsahNo ratings yet
- Group 17 NotesDocument16 pagesGroup 17 NotesChaitan TuduNo ratings yet
- PAPER OF Iodometry and Iodimetry TitrationDocument7 pagesPAPER OF Iodometry and Iodimetry TitrationSaraSaraswatyNo ratings yet
- Poshala Srikshith PatelDocument8 pagesPoshala Srikshith PatelnagarajuNo ratings yet
- Nitrogen Cycle ReportDocument26 pagesNitrogen Cycle ReportDom GudezNo ratings yet
- The Nitrogen CycleDocument3 pagesThe Nitrogen CycleBon Joey J. BernestoNo ratings yet