Professional Documents
Culture Documents
Mole Conversions Practice Answer Key
Uploaded by
api-3762819620 ratings0% found this document useful (0 votes)
52 views1 pageOriginal Title
mole conversions practice answer key
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
52 views1 pageMole Conversions Practice Answer Key
Uploaded by
api-376281962Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
Mole Conversions Practice Answer Key
Molar Mass - Calculate the Molar Mass for the following.
1. What is the molar mass of Magnesium metal?
2. Calculate the molar mass of NaCl
Nq: Q.J.99D"'" I = oi.;>.oiq o
c1:35,'\53x\:; 35.-452> _
)L5s. -143 � 1: J
3. What is the molar mass of NaOH?
Nq-. I >( ;2J.9'i O -=- dJ.CfO, D
0: I X 15,"l'l'I " l':).'lqq
H : I x 1. oo a ; , . oor
)l3'1.'1"17 �
Y>'U> _
il
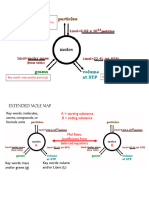
1-Step Conversions- Calculate the following the conversions.Use your Mole Map to help you!
I;
1. Determine the number of atoms in 0.50 moles He.
0.5 m-o\ c..oJ" '0
:><. _
He
.)3
°'b.s He
I mo l H-e
2. Determine the number of moles in 80.00 g 02
O·--l -1--11:,.0)qq =- 31.9C,� 1/
t>-\() I
Yh-Ol =3L9qs
==> ,
3. Determine the volume of 0.24 mol of NO2. ;J-
0 . .;l L.j rl'\.-01 NO� .):l. 4 L- NoJ. Ti
-:::.L5-37fo L.
X "\
\�\ NO� NO�j
4. Determine the number of moles in 7.5 x 10 19 formula units of KNO3
7.5J<JD'" -1.tA. \(f\)03 I l'h-01 KNO;\
)(. I' =13 � v..10 � I X -f..(.;\
-4
1e>.O� 1-lb -t,<..t �,..., � , •
I. .;,y 58 '0 KNo
3
&J
5. Determine the number of moles in 202.22g KNO3
K : I -;. Jc:y, a-:\� = 3"f,09 8 .:>o.;i -hl½ �No3 I�, ktv�
1
t-J: 1 "I- f 'i. 001 :::: ,�. 007 ) IOI. /OJ /Yl"-0 I
fj >< I 01. 10.;),
0: 3" ,s.c:,qC\ :::: in. 9.:,7 --, ,�, = IO1-IOJ
6. Determine the number of moles in 32.5L of N 2 at STP � rKN°J:
\ �I N - -::: } 2 .OD ""'°' � No)
3..1-5 L N� � .- a
-=- {1. � s.; I � I N .)
J
7. Determine the number of moles in 3.4 x 1026 formula units of NaCl.
-7.J.Y L N i). --
3.'I >'lo "'
;>
+. u. �o.CI x I �I No. C. (
3
fo.o:J -,c.10 ;;) -f.u.
8. Determine the moles in 36.SL of carbon monoxide
N o-. C/
3�.5 L CO l�I C.O
X
U.4 L C.O
You might also like
- N Levels Chemistry Notes - Combined ChemistryDocument44 pagesN Levels Chemistry Notes - Combined ChemistryMarcusNg89% (28)
- Solution Manual for The Elements of Polymer Science and EngineeringFrom EverandSolution Manual for The Elements of Polymer Science and EngineeringRating: 4 out of 5 stars4/5 (3)
- AVOGADRO'S LAW Lesson PlanDocument6 pagesAVOGADRO'S LAW Lesson Planhorace hernandez100% (3)
- Solution Manual For Engineering Fluid Mechanics 11th Edition by ElgerDocument61 pagesSolution Manual For Engineering Fluid Mechanics 11th Edition by Elger류재연No ratings yet
- Computations - OR Sep 7, 2022Document2 pagesComputations - OR Sep 7, 2022Hera ArgeiaNo ratings yet
- Mole Conversions Extra Practice Answer KeyDocument1 pageMole Conversions Extra Practice Answer Keyapi-376281962No ratings yet
- Stoichiometry Practice Answer KeyDocument1 pageStoichiometry Practice Answer Keyapi-376281962No ratings yet
- Pset10 Soln PDFDocument6 pagesPset10 Soln PDFBabasrinivas GuduruNo ratings yet
- AppendixesDocument19 pagesAppendixesjeff avecenixNo ratings yet
- Real Numbers Formula NotesDocument2 pagesReal Numbers Formula Notesfelesi2539No ratings yet
- F Dwed Fli R: LK Ol of ofDocument4 pagesF Dwed Fli R: LK Ol of ofLynn ShiyayoNo ratings yet
- EXP09Document7 pagesEXP09Dumishka MadhumalNo ratings yet
- AccountsDocument20 pagesAccountsJasleen KaurNo ratings yet
- Limiting Reactants Extra Practice Answer KeyDocument2 pagesLimiting Reactants Extra Practice Answer Keyapi-376281962No ratings yet
- Ilovepdf MergedDocument4 pagesIlovepdf MergedJose joel suncionNo ratings yet
- Tugas Peluruhan Beta (Fisika Inti) 14-3-2017Document2 pagesTugas Peluruhan Beta (Fisika Inti) 14-3-2017Kartini Sri AstutiNo ratings yet
- Kinetics Refresher WS Answer KeyDocument2 pagesKinetics Refresher WS Answer KeyIchwan Permana MadaniNo ratings yet
- Chem 400 Prereq RVW KeyDocument9 pagesChem 400 Prereq RVW KeyMiguel A. ChávezNo ratings yet
- Experiment No. 4 Energy Gap: TejjjpDocument4 pagesExperiment No. 4 Energy Gap: Tejjjpname nameNo ratings yet
- Molc Os Qa Occupie: O2 Alculate The Va Lume - and Mass O OccupiesDocument6 pagesMolc Os Qa Occupie: O2 Alculate The Va Lume - and Mass O OccupiesAishwarya SarodeNo ratings yet
- HW6 SolutionDocument5 pagesHW6 SolutionAlwalid BaghdadiNo ratings yet
- Ancient Indian Mathematics Part 2Document116 pagesAncient Indian Mathematics Part 2MSHYDERABAD4334No ratings yet
- Admission: Duet Dhaka University of Engineering & Technology Department: Mechanical, CseDocument6 pagesAdmission: Duet Dhaka University of Engineering & Technology Department: Mechanical, CseMd Sohrab Hossain SohelNo ratings yet
- Kinetics ReviewDocument5 pagesKinetics Reviewhareesha015No ratings yet
- Wfr,.,'l... : C:Q: Ba - Sh-!etc:. BirDocument6 pagesWfr,.,'l... : C:Q: Ba - Sh-!etc:. BirsadııqcalıNo ratings yet
- Solution - Manual - Fundamentals - o 30Document1 pageSolution - Manual - Fundamentals - o 30Lit Pao WongNo ratings yet
- File 0001Document8 pagesFile 0001moazzemhosenNo ratings yet
- Sample Question Paper 4 (Explanation)Document23 pagesSample Question Paper 4 (Explanation)prabhakarchsurasiya1234No ratings yet
- Slope DeflexionDocument98 pagesSlope DeflexionlRuth Ramos HuancaNo ratings yet
- Forrc: Lop UDocument1 pageForrc: Lop URoviclopezNo ratings yet
- VTU Exam Question Paper With Solution of 18ME61 Finite Element Methods Aug-2022-Prashant S. HattiDocument53 pagesVTU Exam Question Paper With Solution of 18ME61 Finite Element Methods Aug-2022-Prashant S. HattiFusion gaming100% (1)
- hw6t OptimizationDocument4 pageshw6t Optimizationsamchen984No ratings yet
- ZN Ni CR: Eng. I Treatrnent AnbariDocument13 pagesZN Ni CR: Eng. I Treatrnent AnbariRahul GuptaNo ratings yet
- Civil Engineering Math PDFDocument6 pagesCivil Engineering Math PDFPronab Kumar DasNo ratings yet
- Fundamentals Of: Fifth GmosDocument1 pageFundamentals Of: Fifth GmospatilrajucNo ratings yet
- Esi - Ji Hecct: Ben - T 1 atDocument5 pagesEsi - Ji Hecct: Ben - T 1 atyaseen sheikhNo ratings yet
- موضوعين في الفيزياء 2 ثانويDocument4 pagesموضوعين في الفيزياء 2 ثانويAmir MirouNo ratings yet
- Mathematics Lab Manual (Activity 1)Document5 pagesMathematics Lab Manual (Activity 1)Heeral SinghNo ratings yet
- Finalexam SolutionsDocument8 pagesFinalexam SolutionsJonah BallNo ratings yet
- Timber and Steel - Bending and Shearing Stress of BeamsDocument2 pagesTimber and Steel - Bending and Shearing Stress of BeamsPrincess Lyka LopezNo ratings yet
- Ts Eamcet Engg Qp2017Document61 pagesTs Eamcet Engg Qp2017AyeshaNo ratings yet
- 2014 Main MarkSheet PDFDocument58 pages2014 Main MarkSheet PDFJivesh QuereshiNo ratings yet
- Lusiana Nurhayati Siregar Tugas 2 Bandar UdaraDocument5 pagesLusiana Nurhayati Siregar Tugas 2 Bandar UdaraLusiana Nurhayati SiregarNo ratings yet
- O-Levels Mathematics 2013Document37 pagesO-Levels Mathematics 2013sazinithelma8No ratings yet
- Kannada Question Paper 2022Document11 pagesKannada Question Paper 2022Kauser JahanNo ratings yet
- Scan 26 Ago. 2018 PDFDocument3 pagesScan 26 Ago. 2018 PDFAlberto Lazo ManriqueNo ratings yet
- Red! KJ (1: Fy RJDocument4 pagesRed! KJ (1: Fy RJMuhammad Rizwan QureshiNo ratings yet
- MIT3 044S13 2012exam2solnsDocument8 pagesMIT3 044S13 2012exam2solnsDak KaizNo ratings yet
- Scan May 5, 2020 PDFDocument5 pagesScan May 5, 2020 PDFKampret 28No ratings yet
- Geometry Test 9 ReviewDocument5 pagesGeometry Test 9 ReviewzNo ratings yet
- Quiz3 KeyDocument2 pagesQuiz3 KeyRicardo Andres HindsNo ratings yet
- TTJ/JV-: Result Sheet - Experiment No. 2 (Characteristic of Venturi Meter)Document7 pagesTTJ/JV-: Result Sheet - Experiment No. 2 (Characteristic of Venturi Meter)Muhd AdamNo ratings yet
- Exam 2 S12 SolDocument9 pagesExam 2 S12 SolAriane Jill HipolitoNo ratings yet
- Noted For Processs - 43Document109 pagesNoted For Processs - 43NamernamesNo ratings yet
- Scan - Parcial LXDocument5 pagesScan - Parcial LXGabriela NiñoNo ratings yet
- Signals Systems Jan 2014 PDFDocument2 pagesSignals Systems Jan 2014 PDFKomalNo ratings yet
- Bolivar, Rojas - Tarea1Document11 pagesBolivar, Rojas - Tarea1Angela Patricia Rojas MottaNo ratings yet
- Corriges 2Document2 pagesCorriges 2MaishaNo ratings yet
- CRE Exp2Document3 pagesCRE Exp2kabali007123No ratings yet
- مصدر 8Document15 pagesمصدر 8Alaa AskNo ratings yet
- GPC SD Prob 1Document4 pagesGPC SD Prob 1B Darshan G CV026No ratings yet
- Ion Beams for Materials AnalysisFrom EverandIon Beams for Materials AnalysisR. Curtis BirdNo ratings yet
- Tables of Coefficients for the Analysis of Triple Angular Correlations of Gamma-Rays from Aligned NucleiFrom EverandTables of Coefficients for the Analysis of Triple Angular Correlations of Gamma-Rays from Aligned NucleiNo ratings yet
- Interactive Simulation RubricDocument1 pageInteractive Simulation Rubricapi-376281962No ratings yet
- Limiting Reactants Notes HandoutDocument9 pagesLimiting Reactants Notes Handoutapi-376281962No ratings yet
- Interactive Simulation RubricDocument1 pageInteractive Simulation Rubricapi-376281962No ratings yet
- Limiting Reactants Extra Practice Answer KeyDocument2 pagesLimiting Reactants Extra Practice Answer Keyapi-376281962No ratings yet
- Gummi Bear Poster RubricDocument2 pagesGummi Bear Poster Rubricapi-376281962No ratings yet
- Stoichiometry Notes HandoutDocument9 pagesStoichiometry Notes Handoutapi-376281962No ratings yet
- Gummy Bear InstructionsDocument1 pageGummy Bear Instructionsapi-376281962No ratings yet
- Mole Map Handout UploadDocument1 pageMole Map Handout Uploadapi-376281962No ratings yet
- Limiting Reactants Extra PracticeDocument2 pagesLimiting Reactants Extra Practiceapi-376281962No ratings yet
- Mole Map Handout UploadDocument1 pageMole Map Handout Uploadapi-376281962No ratings yet
- Edpuzzle Lecture RubricDocument1 pageEdpuzzle Lecture Rubricapi-376281962No ratings yet
- Edpuzzle Lecture RubricDocument1 pageEdpuzzle Lecture Rubricapi-376281962No ratings yet
- Assessment RubricDocument1 pageAssessment Rubricapi-376281962No ratings yet
- Dimensional Analysis Extra PracticeDocument1 pageDimensional Analysis Extra Practiceapi-376281962No ratings yet
- Dimensional Analysis Extra Practice Answer KeyDocument3 pagesDimensional Analysis Extra Practice Answer Keyapi-376281962No ratings yet
- Naming Review Practice Answer KeyDocument1 pageNaming Review Practice Answer Keyapi-376281962No ratings yet
- Edpuzzle Lecture RubricDocument1 pageEdpuzzle Lecture Rubricapi-376281962No ratings yet
- SyllabusDocument2 pagesSyllabusapi-376281962No ratings yet
- Discussion RubricDocument1 pageDiscussion Rubricapi-376281962No ratings yet
- Gen Chem 1 Module 3 2nd Edition 2021Document16 pagesGen Chem 1 Module 3 2nd Edition 2021jonzebedeeNo ratings yet
- 16.2 Concentrations of SolutionsDocument55 pages16.2 Concentrations of SolutionsAlejandro SuazoNo ratings yet
- CHPT 12.2 PowerpointDocument62 pagesCHPT 12.2 PowerpointA ANo ratings yet
- Phys2 Ch4 Kineticsgas NewDocument76 pagesPhys2 Ch4 Kineticsgas NewQuỳnh NguyễnNo ratings yet
- 6th ChapDocument15 pages6th ChapAYESHA MUMTAZNo ratings yet
- Molarity QuestionsDocument9 pagesMolarity QuestionsNur MahammadNo ratings yet
- Concentration Terms Formula PDFDocument3 pagesConcentration Terms Formula PDFShiven MundraNo ratings yet
- Enjoy Chemistry: Some Basic Concepts of ChemistryDocument148 pagesEnjoy Chemistry: Some Basic Concepts of ChemistryWaqas KhanNo ratings yet
- Mole Concept and StoichiometryDocument6 pagesMole Concept and StoichiometrySamridhi DasNo ratings yet
- Learning Advanced Chemistry: Quarter 2 - Week 4 and 5Document7 pagesLearning Advanced Chemistry: Quarter 2 - Week 4 and 5Aliah Jeonelle RamosNo ratings yet
- 3.4 The Mole and The Volume of GasDocument37 pages3.4 The Mole and The Volume of GasSiva GuruNo ratings yet
- Arihant Ncert Exemplar Chemistry Solution Class 11Document313 pagesArihant Ncert Exemplar Chemistry Solution Class 11Md Kutubuddin Sardar100% (1)
- Chemistry Form 6 Chap 01 PDFDocument44 pagesChemistry Form 6 Chap 01 PDFryder1man6433No ratings yet
- STPM Sem 1 Introductory Class Notes 2020Document12 pagesSTPM Sem 1 Introductory Class Notes 2020Johnson116No ratings yet
- 3 Konsep Mol, Formula Dan Persamaan Kimia: Mole Concept, Formulae and Chemical EquationsDocument11 pages3 Konsep Mol, Formula Dan Persamaan Kimia: Mole Concept, Formulae and Chemical EquationsAdibsyabil Bin Muhamad FirdausNo ratings yet
- Science 9 q2 Mod8 Percentage-composition-Of-compounds VerfinalDocument28 pagesScience 9 q2 Mod8 Percentage-composition-Of-compounds VerfinalAbel Emmanuel Solitario Cabrales100% (1)
- Chapter 5 GasesDocument74 pagesChapter 5 GasesReem HamadNo ratings yet
- Tips Edited by Mr. Chai and Yati 2016 PDFDocument10 pagesTips Edited by Mr. Chai and Yati 2016 PDFsitiNo ratings yet
- Topic 1 Stoichiometric Relationships PDFDocument7 pagesTopic 1 Stoichiometric Relationships PDFDiksha SinghNo ratings yet
- 2010 Chemistry Stage 3 Sample Marking KeyDocument32 pages2010 Chemistry Stage 3 Sample Marking KeyMichael BobNo ratings yet
- General Organic and Biological Chemistry 2nd Edition Janice Gorzynski Smith Solutions Manual 1Document36 pagesGeneral Organic and Biological Chemistry 2nd Edition Janice Gorzynski Smith Solutions Manual 1amynash23052000xne100% (24)
- 5.1 Mole WorksheetDocument4 pages5.1 Mole Worksheetzhenrong zhaoNo ratings yet
- Arihant 36 Years Chapterwise Solutions NEET Chemistry 2022Document254 pagesArihant 36 Years Chapterwise Solutions NEET Chemistry 2022ashiayan xeroxNo ratings yet
- HSC Chemistry NotesDocument5 pagesHSC Chemistry NotesananyahatesithereNo ratings yet
- StochiometryDocument20 pagesStochiometryongkikoNo ratings yet
- Dalton's Law (Law of Partial Pressures)Document3 pagesDalton's Law (Law of Partial Pressures)Luyando LikubangwaNo ratings yet
- Chemistry 2Document25 pagesChemistry 2try to smileNo ratings yet