Professional Documents
Culture Documents
Nomenclature Ans
Uploaded by
dlc352-sc1Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Nomenclature Ans
Uploaded by
dlc352-sc1Copyright:
Available Formats
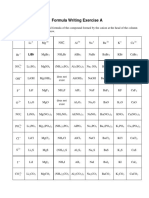
1. Give the correct names for each of the compounds listed below.
a) NaCl sodium chloride n) ZrS2 zirconium sulfide
b) FrBr francium bromide o) AgI silver iodide
c) KF potassium fluoride p) BaSe barium selenide
d) RaS radium sulfide q) MgO magnesium oxide
e) LiI lithium iodide r) LaBr3 lanthanum bromide
f) Li3N lithium nitride s) Sr3N2 strontium nitride
g) AlBr3 aluminum bromide t) Cd3As2 cadmium arsenide
h) CdCl2 cadmium chloride u) Rb2Se rubidium selenide
i) K2O potassium oxide v) Rb3N rubidium nitride
j) InF3 indium fluoride w) BaF2 barium fluoride
k) ZnO zinc oxide x) ZrTe2 zirconium telluride
l) Y2O3 yttrium oxide y) Cs3P cesium phosphide
m) CaTe calcium telluride z) Y2O3 yttrium oxide
2. Write the correct chemical formula for each of the following compounds.
a) potassium bromide KBr n) potassium nitride K3N
b) zinc bromide ZnBr2 o) aluminum bromide AlBr3
c) lithium iodide LiI p) zinc phosphide Zn3P2
d) scandium chloride ScCl3 q) magnesium sulfide MgS
e) magnesium chloride MgCl2 r) hafnium chloride HfCl4
f) magnesium oxide MgO s) barium sulfide BaS
g) hydrogen sulfide H2S t) tantalum oxide Ta2O5
h) gallium iodide GaI3 u) zirconium nitride Zr3N4
i) sodium oxide Na2O v) potassium selenide K2Se
j) magnesium selenide MgSe w) germanium fluoride GeF4
k) calcium fluoride CaF2 x) francium phosphide Fr3P
l) aluminum oxide Al2O3 y) zinc arsenide Zn3As2
m) beryllium chloride BeCl2 z) scandium telluride Sc2Te3
L. h. s. – Chemistry – Nomenclature – Answers – Page 1
3. Give the correct names for each of the compounds listed below.
a) CaSO4 calcium sulfate n) Ta(IO3)5 tantalum iodate
b) Ca3(AsO4)2 calcium arsenate o) (NH4)3PO4 ammonium phosphate
c) NH4Cl ammonium chloride p) AgClO silver hypochlorite
d) Mg3(AsO3)2 magnesium arsenite q) KOH potassium hydroxide
e) NaC2H3O2 sodium acetate r) NaC8H11N2O3 sodium barbital
f) NaOCN sodium cyanate s) HNO3 hydrogen nitrate
g) Al2(SO4)3 aluminum sulfate t) In(VO3)3 indium vandate
h) K2Cr2O7 potassium dichromate u) Na2HPO3 sodium hydrogen phosphite
i) NH4NO3 ammonium nitrate v) Ta2(TeO4)5 tantalum tellurate
j) KSCN potassium thiocyanate w) Ca(NO)2 calcium hyponitrite
k) Al(OH)3 aluminum hydroxide x) Zn(VO3)2 zinc vandate
l) MgS2O8 magnesium peroxydisulfate y) Ba(OH)2 barium hydroxide
m) NaHCO3 sodium bicarbonate or z) CaC8H4O4 calcium phthalate
sodium hydrogen carbonate
4. Write the correct chemical formula for each of the following compounds.
a) sodium acetate NaC2H3O2 n) silver fluorite AgFO2
b) aluminum tetraborate Al2(B4O7)3 o) scandium hydroxide Sc(OH)3
c) calcium bromate Ca(BrO3)2 p) aluminum citrate AlC6H5O7
d) sodium silicate Na2SiO3 q) hafnium nitrate Hf(NO3)4
e) magnesium citrate Mg3(C6H5O7)2 r) francium hydrogen oxalate FrHC2O4
f) calcium tungstate CaWO4 s) rubidium permanganate RbMnO4
g) potassium cyanide KCN t) gallium sulfite Ga2(SO3)3
h) zinc phthalate ZnC8H4O4 u) ammonium dichromate (NH4)2Cr2O7
i) barium carbonate BaCO3 v) cesium hypochlorite CsClO
j) indium stearate In(C17H35COO)3 w) sodium phosphite Na3PO3
k) calcium dichromate CaCr2O7 x) sodium dihydrogen phosphate NaH2PO4
l) yttrium tripolyphosphite Y5(P3O10)3 y) sodium hydrogen phosphate Na2HPO4
m) zirconium bicarbonate Zr(HCO3)4 z) zirconium uranate Zr(UO4)4
L. h. s. – Chemistry – Nomenclature – Answers – Page 2
5. Give the correct names for each of the compounds listed below.
a) FeI3 iron(III) iodide m) WO3 tungsten(VI) oxide
ferric iodide
n) PuPO4 plutonium(III) phosphate
b) Bi2(SO4)3 bismuth(III) sulfate
o) PdI4 palladium(IV) iodide
c) FeI2 iron(II) iodide
ferrous iodide p) Os(NO3)4 osmium(IV) nitrate
d) HgHCO3 mercury(I) bicarbonate q) Co2S3 cobalt(III) sulfide
mercury(I) hydrogen carbonate
r) Ti3N4 titanium(IV) nitride
mercurous bicarbonate
mercurous hydrogen carboate s) MnO2 manganese(IV) oxide
e) NiO nickel(II) oxide
t) NiSO4 nickel(II) sulfate
f) Pb(H2PO3)2 lead(II) dihydrogen phosphite
u) Ti(Cr2O7)2 titanium(IV) dichromate
plumbous dihydrogen phosphite
g) CuBr2 copper(II) bromide v) FeSO3 iron(II) sulfite
cupric bromide ferrous sulfite
h) Pt(CrO4)2 platinum(IV) chromate w) OsS2 osmium(IV) sulfide
i) Cr2O3 chromium(III) oxide x) Hg(NO2)2 mercury(II) nitrite
mercuric nitrite
j) Sb2(SO5)3 antimony(III) persulfate
y) SnSO4 tin(II) sulfate
k) AuCl3 gold(III) chloride stannous sulfate
auric chloride z) AuCl3 gold(III) chloride
l) Np(MnO3)5 neptunium(V) manganate auric chloride
6. Write the correct chemical formula for each of the following compounds.
a) lead(IV) oxide PbO2 n) polonium(IV) sulfide PoS2
b) antimony(V) bromite Sb(BrO2)5 o) vanadium(V) iodate V(IO3)5
c) cobalt(II) fluoride CoF2 p) plumbic phosphate Pb3(PO4)4
d) ferric thiosulfate Fe2(S2O3)3 q) molybdenum(VI) benzoate Mo(C6H5COO)6
e) copper(II) cyanide Cu(CN)2 r) niobium(V) oxide Nb2O5
f) stannic tartrate Sn(C4H4O6)2 s) aurous silicate Au2SiO3
g) copper(I) nitride Cu3N t) titanium(IV) sulfite Ti(SO3)2
h) platinum(IV) dichromate Pt(Cr2O7)2 u) cobaltous chloride CoCl2
i) nickel(II) acetate Ni(C2H3O2)2 v) samarium(III) nitrite Sm(NO2)3
j) tin(II) peroxydisulfate SnS2O8 w) plumbic hydroxide Pb(OH)4
k) gallium(III) acetate Ga(C2H3O2)3 x) terbium (IV) periodate Tb(IO4)4
l) gold(III) uranate Au(UO4)3 y) iridium(IV) periodate Ir(IO4)4
m) osmium(IV) sulfate Os(SO4)2 z) stannous bicarbonate Sn(HCO3)2
L. h. s. – Chemistry – Nomenclature – Answers – Page 3
7. Give the correct names for each of the compounds listed below.
a) CS2 carbon disulfide i) PBr5 phosphoruse pentabromide
b) SF2 sulfur difluoride j) N2O4 dinitrogen tetroxide
c) CO carbon monoxide k) SO3 sulfur trioxide
d) ICl3 iodine trichloride l) SO2 sulfur dioxide
e) CCl4 carbon tetrachloride m) N2O3 dinitrogen trioxide
f) As2O3 diarsenic trioxide n) Cl2O dichlorine monoxide
g) PBr3 phosphorus tribromide o) SF6 sulfur hexafluoride
h) IF5 iodine pentafluoride p) SiO2 silicon dioxide
8. Write the correct chemical formula for each of the following compounds.
a) nitrogen monoxide NO i) dinitrogen tetroxide N2O4
b) carbon dioxide CO2 j) diphosphorus trisulfide P2S3
c) iodine monochloride ICl k) chlorine dioxide ClO2
d) sulfur trioxide SO3 l) silicon disulfide SiS2
e) chlorine trifluoride ClF3 m) silicon tetrafluoride SiF4
f) phosphorus pentachloride PCl5 n) sulfur dioxide SO2
g) bromine pentafluoride BrF5 o) tricarbon disulfide C3S2
h) carbon tetrachloride CCl4 p) dinitrogen pentoxide N2O5
L. h. s. – Chemistry – Nomenclature – Answers – Page 4
9. Give the correct names for each of the compounds listed below.
a) Li2SiF6•2H2O lithium hexafluorosilicate dehydrate
b) Na2B4O7•10H2O sodium tetraborate decahydrate
c) MgSO3•6H2O magnesium sulfite hexahydrate
d) NaC2H3O2•3H2O sodium acetate trihydrate
e) CuSO4•5H2O copper(II) sulfate pentahydrate or cupric sulfate pentahydrate
f) MgSO4•9H2O magnesium sulfate nonahydrate
g) CaSO4•2H2O calcium sulfate dihydrate
h) MgCl2•6H2O magnesium chloride hexahydrate
i) FeSO4•7H2O iron(II) sulfate heptahydrate or ferrous sulfate heptahydrate
j) NaHS•H2O sodium hydrogen sulfide monohydrate or sodium bisulfide monohydrate
10. Write the correct chemical formula for each of the following compounds.
a) calcium chloride hexahydrate CaCl2•6H2O
b) barium chloride dihydrate BaCl2•2H2O
c) calcium nitrate tetrahydrate Ca(NO3)2•4H2O
d) sodium chromate tetrahydrate Na2CrO4•4H2O
e) copper(II) nitrate trihydrate Cu(NO3)2•3H2O
f) plumbous acetate trihydrate Pb(C2H3O2)2•3H2O
g) aluminum chloride hexahydrate AlCl3•6H2O
h) sodium dihydrogen phosphate nonahydrate NaH2PO4•9H2O
i) cobalt(II) nitrate hexahydrate Co(NO3)2•6H2O
j) cobaltous sulfate hexahydrate CoSO4•6H2O
L. h. s. – Chemistry – Nomenclature – Answers – Page 5
11. Give the correct formula for each of the compounds listed below.
a) hydrochloric acid HCl(aq) n) hydroiodic acid HI(aq)
b) citric acid H3C6H5O7(aq) o) phosphoric acid H3PO4(aq)
c) benzoic acid HC6H5COO(aq) p) nitrous acid HNO2(aq)
d) acetic acid HC2H3O2(aq) q) thiosulfurous acid H2S2O2(aq)
e) periodic acid HIO4(aq) r) nitric acid HNO3(aq)
f) lactic acid HC3H5O3(aq) s) hydrotelleric acid H2Te(aq)
g) formic acid HCOOH(aq) t) hydrocyanic acid HCN(aq)
h) iodic acid HIO3(aq) u) hydroselenic acid H2Se(aq)
i) oxalic acid H2C2O4(aq) v) nitrous acid HNO2(aq)
j) sulfurous acid H2SO3(aq) w) hypooxalous acid H2C2O2(aq)
k) sulfuric acid H2SO4(aq) x) hydrofluoric acid HF(aq)
l) carbonic acid H2CO3(aq) y) boric acid H3BO3(aq)
m) phosphorous acid H3PO3(aq) z) hydrosulfuric acid H2S(aq)
12. Write the correct name for each of the following compounds.
a) HC2H3O2(aq) acetic acid m) HC5H8NO4(aq) glutamic acid
b) H2B4O7(aq) tetraboric acid n) H3PO4(aq) phosphoric acid
c) H3AsO3(aq) arsenous acid o) HClO(aq) hypochlorous acid
d) HI(aq) hydroiodic acid p) HBr(aq) hydrobromic acid
e) H3BO3(aq) boric acid q) H2C2O4(aq) oxalic acid
f) HF(aq) hydrofluoric acid r) H2CO3(aq) carbonic acid
g) HCNO(aq) cyanic acid s) H2SiO2(aq) silicous acid
h) H2SO4(aq) sulfuric acid t) HFO2(aq) fluorous acid
i) H2C4H4O6(aq) tartric acid u) HC17H35COO(aq) stearic acid
j) HCN(aq) hydrocyanic acid v) H3PO3(aq) phosphorous acid
k) H(HCOO)(aq) formic acid w) HCl(aq) hydrochloric acid
l) HNO3(aq) nitric acid x) HBrO2(aq) bromous acid
L. h. s. – Chemistry – Nomenclature – Answers – Page 6
A. Give the correct chemical formula for each of the following compounds.
1. sodium hydroxide NaOH 35. nickel(II) peracetate Ni(C2H3O3)2
2. copper(II) sulfide CuS 36. mercuric chloride dehydrate HgCl2•2H2O
3. potassium phosphide K3P 37. dinitrogen trioxide N2O3
4. ozone O3 38. sodium hypoiodite NaIO
5. lithium nitride Li3N 39. potassium cyanide KCN
6. lithium hydride LiH 40. potassium aluminum sulfate KAl(SO4)2
7. magnesium percarbonate MgCO4 41. ammonium hypophosphite (NH4)3PO2
8. aluminum sulfite Al2(SO3)3 42. potassium uranate KUO4
9. sodium sulfate heptahydrate Na2SO4•7H2O 43. lithium peroxide Li2O2
10. sodium carbonite Na2CO2 44. perchloric acid HClO4(aq)
11. perchloric acid HClO4(aq) 45. ammonia NH3
12. calcium hyponitrite Ca(NO)2 46. iodous acid HIO2(aq)
13. nitrous acid HNO2(aq) 47. hydrogen peroxide H2O2
14. sulfurous acid H2SO3(aq) 48. gold(III) periodate Au(IO4)3
15. zinc acetate trihydrate Zn(C2H3O2)2•3H2O 49. sodium oxide Na2O
16. potassium hypochromite K2CrO2 50. sodium glutamate NaC5H8NO4
17. barium nitride Ba3N2 51. iron(II) sulfate FeSO4
18. cobalt(II) perphosphate Co3(PO5)2 52. barium perchlorate Ba(ClO4)2
19. carbon dioxide CO2 53. manganese(II) nitrate Mn(NO3)2
20. sulfuric acid H2SO4(aq) 54. osmium(IV) thiosulfate Os(S2O3)2
21. iron(III) chloride FeCl3 55. chromium(III) nitrate Cr(NO3)3
22. chromium(III) acetate Cr(C2H3O2)3 56. boric acid H3BO3(aq)
23. hydrobromic acid HBr(aq) 57. rubidium acetate RbC2H3O2
24. silver carbonate Ag2CO3 58. hypoiodous acid HIO(aq)
25. hydrogen bromide HBr(g) 59. cerium(III) phosphate CePO4
26. barium chloride BaCl2 60. nitrous acid HNO2(aq)
27. boron trifluoride BF3 61. chromium(III) nitride CrN
28. calcium hydroxide Ca(OH)2 62. nitric acid HNO3(aq)
29. calcium hydride CaH2 63. magnesium nitrate Mg(NO3)2
30. lead(II) hyposulfite PbSO2 64. hypoiodous acid HIO(aq)
31. hypophosphorous acid H3PO2(aq) 65. copper(II) tartrate CuC4H4O6
32. carbonic acid H2CO3 66. arsenous acid H3AsO3(aq)
33. beryllium perchlorate Be(ClO4)2 67. magnesium hexafluorosilicate MgSiF6
34. ferrous hydroxide Fe(OH)2 68. cyanic acid HOCN(aq)
L. h. s. – Chemistry – Nomenclature – Answers – Page 7
B. Give the correct names for each of the compounds listed below.
1. SnO2 tin(IV) oxide 35. Na3PO4 sodium phosphate
stannic oxide
36. Na2CrO4 sodium chromate
2. Sb2S3 antimony(III) sulfide
3. HgS mercury(II) sulfide 37. LiClO4 lithium perchlorate
mercuric sulfide 38. Zn(C2H3O)2 zinc acetite
4. MoS2 molybdenum(IV) sulfide 39. Au(CN)3 gold(III) cyanide
5. FeS iron(II) sulfide
40. K2CrO4 potassium chromate
ferrous sulfide
41. KHCO3 potassium bicarbonate
6. HgO mercury(II) oxide
potassium hydrogen carbonate
mercuric oxide
7. AuCl3 gold(III) chloride 42. Mn(OH)2 manganese(II) hydroxide
auric chloride 43. Ba(SCN)2 barium thiocyanate
8. NiBr2 nickel(II) bromide 44. RbCN rubidium cyanide
9. MgO magnesium oxide 45. NaBrO sodium hypobromite
10. NaBr sodium bromide 46. Al2(SO5)3 aluminum persulfate
11. Al2O3 aluminum oxide 47. Fe(ClO)2 iron(II) hypochlorite
12. CaO calcium oxide ferrous hypochlorite
13. Ag2S silver sulfide 48. (NH4)2CO3 ammonium carbonate
14. CaH2 calcium hydride 49. Zn(NO2)2 zinc nitrite
15. K2CO3 potassium carbonate 50. Ca(NO3)2 calcium nitrate
16. (NH4)2S ammonium sulfide 51. NH4OH ammonium hydroxide
17. Cr(NO3)2 chromium(II) nitrate 52. NiPO2 nickel(III) hypophosphite
18. KMnO4 potassium permanganate 53. NH3 ammonia
19. SO3 sulfur trioxide 54. CaSO4 calcium sulfate
20. P2S5 diphosphorus pentasulfide 55. Pb(HSO4)4 lead(IV) hydrogen sulfate
21. As2S3 diarsenic trisulfide plumbic bisulfate
22. CCl4 carbon tetrachloride 56. Ca(ClO3)2 calcium chlorate
23. N2O4 dinitrogen tetroxide 57. AlPO4 aluminum phosphate
24. NO nitrogen monoxide 58. Li2CO2 lithium carbonite
25. H3BO3 boric acid 59. PCl5 phosphorus pentachloride
26. MgSCN magnesium thiocyanate 60. Mg(NO3)2 magnesium nitrate
27. HNO2 nitrous acid 61. SO2 sulfur dioxide
28. As2S5 diarsenic pentasulfide 62. BaCr2O7 barium dichromate
29. H3PO4 phosphoric acid 63. SrH2 strontium hydride
30. Fe(NO3)2 iron(II) nitrate 64. H2SO4 sulfuric acid
ferrous nitrate 65. Na2O2 sodium peroxide
31. H3AsO3 arsenous acid 66. CsH2PO4 cesium dihydrogen phosphate
32. Cu2SO4 copper(I) sulfate 67. Pb3(PO3)2 lead(II) phosphite
cuprous sulfate plumbous phosphite
33. HIO3 iodic acid 68. HBr(aq) hydrobromic acid
34. K2C2O4 potassium oxalate
L. h. s. – Chemistry – Nomenclature – Answers – Page 8
You might also like
- WS#2 Naming and Writing Inorganic CompoundsDocument6 pagesWS#2 Naming and Writing Inorganic CompoundsOw ZeeNo ratings yet
- SaltsDocument2 pagesSaltsignacymarcinkiewicz1No ratings yet
- Chemical Formula Writing WorksheetDocument5 pagesChemical Formula Writing WorksheetÂziz ShuvoNo ratings yet
- Dustin Kern - Writing Polyatomic Names and Formulas WorksheetDocument2 pagesDustin Kern - Writing Polyatomic Names and Formulas Worksheetapi-644456294No ratings yet
- Answers Nomencalture Extra Practice PDFDocument3 pagesAnswers Nomencalture Extra Practice PDFAngel Joy CatalanNo ratings yet
- 2018 Thanksgiving Turkey Cards KeyDocument1 page2018 Thanksgiving Turkey Cards KeyHakkyu KimNo ratings yet
- SCH3U0 Nomenclature PracticeDocument7 pagesSCH3U0 Nomenclature PracticeArmann JohalNo ratings yet
- Naming Ionic Compounds Practice Worksheet - SolutionsDocument3 pagesNaming Ionic Compounds Practice Worksheet - SolutionsJa Son Tonogbanua100% (1)
- Chemical Formula Writing Worksheet SolutionsDocument3 pagesChemical Formula Writing Worksheet SolutionsReid Pineda Creencia100% (1)
- Chapter - 7 Correction Naming CompoundsDocument2 pagesChapter - 7 Correction Naming CompoundsMurad IsayevNo ratings yet
- Namingpacketanswers 3Document14 pagesNamingpacketanswers 3Supremo DelagerNo ratings yet
- Jordan Paddock - Writing Polyatomic Names and Formulas WorksheetDocument1 pageJordan Paddock - Writing Polyatomic Names and Formulas Worksheetapi-675312232No ratings yet
- Activity5 ChemicalformulasDocument2 pagesActivity5 ChemicalformulasJohn Hayden Dela CruzNo ratings yet
- 1.5A Ionic Compounds, Extra ExercisesDocument2 pages1.5A Ionic Compounds, Extra ExercisesDaniel StandringNo ratings yet
- (CHEM) Pairwork Chem NomencDocument1 page(CHEM) Pairwork Chem NomencsodiumboyupinthishoeNo ratings yet
- Nomenclature WorksheetDocument5 pagesNomenclature WorksheetJapphetNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetSam JoNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice Worksheet김동주No ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetBrillantes JYNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetOlivia DitzelNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice Worksheetarlene serdiniaNo ratings yet
- Review 1 WorksheetDocument2 pagesReview 1 WorksheetsupremeNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetBrillantes JYNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetSunshine LadyNo ratings yet
- FormulaDocument6 pagesFormulaLars RembrandtNo ratings yet
- 1.5B Solutions For Ionic Compounds, Extra ExercisesDocument1 page1.5B Solutions For Ionic Compounds, Extra ExercisesDaniel StandringNo ratings yet
- What Is The Systematic Name of The Following Compound (Solved)Document7 pagesWhat Is The Systematic Name of The Following Compound (Solved)Debayanbasu.juNo ratings yet
- Nomenclature Review AssignmentDocument8 pagesNomenclature Review AssignmentTish BarnesNo ratings yet
- 5.7-5.10 Naming Mixed Ionic and Covalent Compounds AnswersDocument2 pages5.7-5.10 Naming Mixed Ionic and Covalent Compounds AnswersAlan MartínNo ratings yet
- Baso Agno H S Cao H Co MG (Po) K Cro NaiDocument2 pagesBaso Agno H S Cao H Co MG (Po) K Cro NaiAmy BalicagNo ratings yet
- Nomenclature Homework 1Document5 pagesNomenclature Homework 1James PerriamNo ratings yet
- Foundations of College Chemistry 14th Edition Hein Solutions Manual DownloadDocument9 pagesFoundations of College Chemistry 14th Edition Hein Solutions Manual DownloadJohn Gaudreau100% (25)
- Max Hyman - Writing Mixed Names and Formulas WorksheetDocument1 pageMax Hyman - Writing Mixed Names and Formulas Worksheetapi-673504994No ratings yet
- Inorganic Compounds: Chemical Name Chemical FormulaDocument6 pagesInorganic Compounds: Chemical Name Chemical FormulaFrendick LegaspiNo ratings yet
- Nomenclature Assignment Part 1Document4 pagesNomenclature Assignment Part 1marNo ratings yet
- 2.4.3 Chemical Formula and Naming Practice QuestionsDocument7 pages2.4.3 Chemical Formula and Naming Practice Questionsphat.vuongNo ratings yet
- Ws Naming Compounds 9-11-08Document2 pagesWs Naming Compounds 9-11-08Yahra Aquino100% (1)
- Inorganic Nomenclature Rules, Examples and Practice Problems Bansal PatternDocument7 pagesInorganic Nomenclature Rules, Examples and Practice Problems Bansal PatternKumarNo ratings yet
- Pcqa111 - Assignment For Nomenclature and Formula WritingDocument1 pagePcqa111 - Assignment For Nomenclature and Formula WritingRusselle Kate AlvaradoNo ratings yet
- IUPAC Name: Give The Chemical Formula For Each of The FollowingDocument1 pageIUPAC Name: Give The Chemical Formula For Each of The FollowingKiki ShofiaNo ratings yet
- Ionic Compounds Worksheet-IiiDocument1 pageIonic Compounds Worksheet-IiitylerNo ratings yet
- نام گذاری ترکیبهای شیمیائیDocument2 pagesنام گذاری ترکیبهای شیمیائیapi-3706290No ratings yet
- Nomenclature Worksheet NDocument2 pagesNomenclature Worksheet NVictor GarciaNo ratings yet
- Unit 04 - Study Guide - ANSWERSDocument2 pagesUnit 04 - Study Guide - ANSWERSBipin GhimireNo ratings yet
- Writing and Naming I: SCIENCE 10 - MAR 3, 2017Document6 pagesWriting and Naming I: SCIENCE 10 - MAR 3, 2017Alfredo L. CariasoNo ratings yet
- 1.6A Molecular Compounds, Extra ExercisesDocument1 page1.6A Molecular Compounds, Extra ExercisesDaniel StandringNo ratings yet
- Answers - Naming Chemical CompoundsDocument3 pagesAnswers - Naming Chemical CompoundsIvy JoyceNo ratings yet
- Problem Set 3 NomenclatureDocument3 pagesProblem Set 3 NomenclatureKê VîňNo ratings yet
- Naming Review Practice Answer KeyDocument1 pageNaming Review Practice Answer Keyapi-376281962No ratings yet
- Formulas and Names of Ionic CompoundsDocument3 pagesFormulas and Names of Ionic CompoundsMandanas GabrielNo ratings yet
- Cation and AnionDocument1 pageCation and Anionsumityadav742008No ratings yet
- Ionic Compound Formula Writing WorksheetDocument2 pagesIonic Compound Formula Writing WorksheetJackson LtorishaNo ratings yet
- Naming N Molar Mass KJJDocument4 pagesNaming N Molar Mass KJJKherulJefriJamenNo ratings yet
- Formula Writing - CambridgeDocument5 pagesFormula Writing - CambridgeQusai Saify100% (3)
- Review #3 NomenclatureDocument1 pageReview #3 NomenclatureCassandra MachadoNo ratings yet
- Nomenclature WorksheetDocument2 pagesNomenclature WorksheetJoseph GagnonNo ratings yet
- Chemical FormulaDocument4 pagesChemical FormulaChii YenNo ratings yet
- The Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyFrom EverandThe Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyNo ratings yet
- The Modern Spanish: Breyer and Zaitsev SystemsFrom EverandThe Modern Spanish: Breyer and Zaitsev SystemsRating: 5 out of 5 stars5/5 (1)
- Kalkulator AB Mix 1Document82 pagesKalkulator AB Mix 1Erwin100% (1)
- Microsoft Word - Chemsheets GCSE 1131 (Reactions of Acids 1) 10.36.19 AMDocument1 pageMicrosoft Word - Chemsheets GCSE 1131 (Reactions of Acids 1) 10.36.19 AMLanbin CuiNo ratings yet
- Quantities and Equation - Worksheet 2 (Writing Equations)Document3 pagesQuantities and Equation - Worksheet 2 (Writing Equations)pusat tuisyen spektrum global jadestarNo ratings yet
- Pemakaian Obat RasionalDocument24 pagesPemakaian Obat RasionalMARTININo ratings yet
- ChemistryDocument44 pagesChemistryRadhe RadheNo ratings yet
- Raw MaterialDocument45 pagesRaw MaterialHr DaniNo ratings yet
- Analytical Chemistry Paper 2Document2 pagesAnalytical Chemistry Paper 2Naveen KumarNo ratings yet
- Salt AnalysisDocument26 pagesSalt AnalysisNikhil MishraNo ratings yet
- Laboratory Periodic Table: Gsci1103L-General Chemistry 1 LabDocument6 pagesLaboratory Periodic Table: Gsci1103L-General Chemistry 1 LabAndrea AurielleNo ratings yet
- Naming & Balancing Chemical Formula - Sheet1Document1 pageNaming & Balancing Chemical Formula - Sheet1arseniy kraschenkoNo ratings yet
- PiperPlot QWDocument21 pagesPiperPlot QWAndrés Eduardo Escare RuminotNo ratings yet
- 15 How To Write Salt Analysis Salt No 1,2,3,4Document26 pages15 How To Write Salt Analysis Salt No 1,2,3,4Diluv JayantNo ratings yet
- Chrmistry - STD 10 - Hydrogen ChlorideDocument14 pagesChrmistry - STD 10 - Hydrogen ChlorideMohit NaiduNo ratings yet
- Naming Compounds WorksheetDocument5 pagesNaming Compounds Worksheet吴蔓华No ratings yet
- Flowsheet Aluminium Ammonium SulfatDocument1 pageFlowsheet Aluminium Ammonium SulfatreyhanNo ratings yet
- Penawaran Harga: BrandDocument4 pagesPenawaran Harga: Brandmuhammad rizkyNo ratings yet
- Worksheet - Balancing Equations 1Document2 pagesWorksheet - Balancing Equations 1Shadae ClarkeNo ratings yet
- Properties of CompoundsDocument15 pagesProperties of CompoundsPrasad YarraNo ratings yet
- Limit Tests: Aim: Forms of Chlorides: Apparatus RequiredDocument2 pagesLimit Tests: Aim: Forms of Chlorides: Apparatus RequiredHakka MarwadiNo ratings yet
- FGT 09Document1 pageFGT 09Juned VhoraNo ratings yet
- Scheme of Salt AnalysisDocument6 pagesScheme of Salt AnalysisAntony KonikaraNo ratings yet
- 05 29 92Document26 pages05 29 92antonioNo ratings yet
- Hsslive-xii-chemistry-lab-Scheme For Salt Analysis Simplified For 2020-21Document2 pagesHsslive-xii-chemistry-lab-Scheme For Salt Analysis Simplified For 2020-21Athul SNo ratings yet
- Types of Reactions Worksheet THEN Balancing!Document4 pagesTypes of Reactions Worksheet THEN Balancing!Emil HerreraNo ratings yet
- Excel Meracik Nutrisi Bandung 11 Feb 2018Document30 pagesExcel Meracik Nutrisi Bandung 11 Feb 2018Ariev WahyuNo ratings yet
- Chromium: Chemical Properties of Chromium (1) Reaction With AirDocument13 pagesChromium: Chemical Properties of Chromium (1) Reaction With AirDaniel SuubiNo ratings yet
- Terrorist HandbookDocument63 pagesTerrorist HandbookOscar FrizziNo ratings yet
- Salt Analysis-Ferric ChlorideDocument3 pagesSalt Analysis-Ferric ChlorideVandana0% (1)
- Term 3 Experiment No 3 Law of Conservation of MassDocument3 pagesTerm 3 Experiment No 3 Law of Conservation of MassAditya KhandujaNo ratings yet
- Registrasi Msds Labaux 221117 081835Document4 pagesRegistrasi Msds Labaux 221117 081835Fajar ApriantoNo ratings yet