Professional Documents
Culture Documents
1.5A Ionic Compounds, Extra Exercises
Uploaded by
Daniel Standring0 ratings0% found this document useful (0 votes)
11 views2 pagesCopyright
© © All Rights Reserved
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
11 views2 pages1.5A Ionic Compounds, Extra Exercises
Uploaded by
Daniel StandringCopyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
You are on page 1of 2
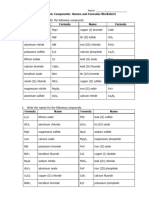
Student Worksheet LSM 1.
5A
Ionic Compounds, Extra Exercises
1. Write the formulas for the following
compounds.
(a) magnesium oxide 2. Write the names for the following
compounds.
(b) sodium fluoride (a) Li2O
(c) aluminium nitride (b) AlCl3
(d) potassium sulfide (c) MgS
(e) lithium iodide (d) CaO
(f) calcium bromide (e) KBr
(g) beryllium oxide (f) BeF
(h) nickel chloride (g) Na3N
(i) magnesium nitride (h) Al2O3
(j) aluminium sulfide (i) CuCl2
(k) copper(I) bromide (j) FeBr3
(l) tin(II) iodide (k) PbS
(m) iron(III) chloride (l) SnO2
(n) calcium phosphide (m) Na2S
(o) lead(II) oxide (n) Mg3P2
(p) lead(IV) fluoride (o) NiO
(q) tin(IV) bromide (p) CuI
(r) copper(II) sulfide (q) PbCl4
(s) iron(II) oxide (r) FeP
(t) calcium nitride (s) CaF2
(t) K3P
36 Review Unit Lab and Study Masters Copyright © 2007 Thomson Nelson
You might also like
- Foundations of College Chemistry 14th Edition Hein Solutions Manual DownloadDocument9 pagesFoundations of College Chemistry 14th Edition Hein Solutions Manual DownloadJohn Gaudreau100% (25)
- Nomenclature WorksheetDocument5 pagesNomenclature WorksheetJapphetNo ratings yet
- Chemical Formula Writing Worksheet SolutionsDocument3 pagesChemical Formula Writing Worksheet SolutionsReid Pineda Creencia100% (1)
- IB Chemistry-Revision On Naming Ionic CompoundsDocument3 pagesIB Chemistry-Revision On Naming Ionic CompoundsRania ShabanNo ratings yet
- Gallium Nitride: Ferric Sulfide Copper SelenideDocument3 pagesGallium Nitride: Ferric Sulfide Copper SelenideFernando CastilloNo ratings yet
- 1.5B Solutions For Ionic Compounds, Extra ExercisesDocument1 page1.5B Solutions For Ionic Compounds, Extra ExercisesDaniel StandringNo ratings yet
- Ionic Compound Formula Writing WorksheetDocument2 pagesIonic Compound Formula Writing WorksheetJackson LtorishaNo ratings yet
- 5.7 Ionic Compounds WorksheetDocument2 pages5.7 Ionic Compounds Worksheetjadattle05No ratings yet
- Ionic Compounds Names and Formulas Worksheet AnswersDocument2 pagesIonic Compounds Names and Formulas Worksheet AnswersShayan UzzamanNo ratings yet
- Post 5.9. Ionic Compounds Practice - ANSWERSDocument3 pagesPost 5.9. Ionic Compounds Practice - ANSWERSAlan MartínNo ratings yet
- WS#2 Naming and Writing Inorganic CompoundsDocument6 pagesWS#2 Naming and Writing Inorganic CompoundsOw ZeeNo ratings yet
- SaltsDocument2 pagesSaltsignacymarcinkiewicz1No ratings yet
- Nomenclature WorksheetDocument2 pagesNomenclature WorksheetJoseph GagnonNo ratings yet
- 2.4.3 Chemical Formula and Naming Practice QuestionsDocument7 pages2.4.3 Chemical Formula and Naming Practice Questionsphat.vuongNo ratings yet
- Chemical Formula Writing WorksheetDocument5 pagesChemical Formula Writing WorksheetÂziz ShuvoNo ratings yet
- Nomenclature Review AssignmentDocument8 pagesNomenclature Review AssignmentTish BarnesNo ratings yet
- Nomenclature AnsDocument8 pagesNomenclature Ansdlc352-sc1No ratings yet
- Review #3 NomenclatureDocument1 pageReview #3 NomenclatureCassandra MachadoNo ratings yet
- Chemical Formula Binary Ionic CompoundsDocument2 pagesChemical Formula Binary Ionic CompoundsRamisNo ratings yet
- 1.6A Molecular Compounds, Extra ExercisesDocument1 page1.6A Molecular Compounds, Extra ExercisesDaniel StandringNo ratings yet
- Bonding WorksheetDocument1 pageBonding WorksheetPuffsNo ratings yet
- Nomenclature Worksheet NDocument2 pagesNomenclature Worksheet NVictor GarciaNo ratings yet
- Activity5 ChemicalformulasDocument2 pagesActivity5 ChemicalformulasJohn Hayden Dela CruzNo ratings yet
- Formulas and Names of Ionic CompoundsDocument3 pagesFormulas and Names of Ionic CompoundsMandanas GabrielNo ratings yet
- WKS001 010 636149 PDFDocument2 pagesWKS001 010 636149 PDFjulsNo ratings yet
- Ionic Compounds Worksheet-IiiDocument1 pageIonic Compounds Worksheet-IiitylerNo ratings yet
- Nomenclature Assignment Part 1Document4 pagesNomenclature Assignment Part 1marNo ratings yet
- Test 1 Formula of IonsDocument6 pagesTest 1 Formula of IonsSEAW FUI MINGNo ratings yet
- Chemical Formula WorksheetDocument4 pagesChemical Formula WorksheetMaria adeelNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice Worksheetarlene serdiniaNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetSam JoNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetSunshine LadyNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice Worksheet김동주No ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetBrillantes JYNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetBrillantes JYNo ratings yet
- Review 1 WorksheetDocument2 pagesReview 1 WorksheetsupremeNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetOlivia DitzelNo ratings yet
- Inorganic Compounds: Chemical Name Chemical FormulaDocument6 pagesInorganic Compounds: Chemical Name Chemical FormulaFrendick LegaspiNo ratings yet
- FormulaDocument6 pagesFormulaLars RembrandtNo ratings yet
- Ws Naming Compounds 9-11-08Document2 pagesWs Naming Compounds 9-11-08Yahra Aquino100% (1)
- Naming Ionic Compounds Practice Worksheet - SolutionsDocument3 pagesNaming Ionic Compounds Practice Worksheet - SolutionsJa Son Tonogbanua100% (1)
- Nomenclature Exercise AnswersDocument3 pagesNomenclature Exercise AnswersAh TseNo ratings yet
- Chapter - 7 Correction Naming CompoundsDocument2 pagesChapter - 7 Correction Naming CompoundsMurad IsayevNo ratings yet
- 2018 Thanksgiving Turkey Cards KeyDocument1 page2018 Thanksgiving Turkey Cards KeyHakkyu KimNo ratings yet
- Namingpacketanswers 3Document14 pagesNamingpacketanswers 3Supremo DelagerNo ratings yet
- Chemical Formula Writing Worksheet2Document2 pagesChemical Formula Writing Worksheet2عابدهعلي100% (1)
- Ionic Compound Naming KeyDocument1 pageIonic Compound Naming Keynisrina amaliaNo ratings yet
- Honors Chemistry WKSHT Names and Formulas V and ANSWERSDocument2 pagesHonors Chemistry WKSHT Names and Formulas V and ANSWERSkijijisellerNo ratings yet
- Ella ScienceDocument2 pagesElla ScienceLorna Ojarliza AchaNo ratings yet
- Writing & Naming Ionic FormulaeDocument1 pageWriting & Naming Ionic FormulaeAnonymousNo ratings yet
- Baso Agno H S Cao H Co MG (Po) K Cro NaiDocument2 pagesBaso Agno H S Cao H Co MG (Po) K Cro NaiAmy BalicagNo ratings yet
- Chemical Formula - Oxidation NumberDocument47 pagesChemical Formula - Oxidation NumberZheng JoeyNo ratings yet
- More Extra Nomenclature Practice - KEYDocument10 pagesMore Extra Nomenclature Practice - KEYelitzelmartinez21No ratings yet
- Problem Set 3 NomenclatureDocument3 pagesProblem Set 3 NomenclatureKê VîňNo ratings yet
- Dustin Kern - Writing Polyatomic Names and Formulas WorksheetDocument2 pagesDustin Kern - Writing Polyatomic Names and Formulas Worksheetapi-644456294No ratings yet
- Ionic CompoundsDocument1 pageIonic CompoundsRoe JoganNo ratings yet
- (CHEM) Pairwork Chem NomencDocument1 page(CHEM) Pairwork Chem NomencsodiumboyupinthishoeNo ratings yet
- The Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyFrom EverandThe Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyNo ratings yet
- Structure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsFrom EverandStructure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsNo ratings yet