Professional Documents
Culture Documents
Writing & Naming Ionic Formulae
Uploaded by
Anonymous0 ratings0% found this document useful (0 votes)
22 views1 pageThe document provides information about writing ionic formulas for ionic compounds. It begins by listing common binary ionic compounds and their formulas, noting they all end in -ide. It then discusses compounds containing complex ions, explaining the roman numerals in parentheses indicate the metal's valency. Finally, it lists the names of additional ionic compounds when given their formulas.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document provides information about writing ionic formulas for ionic compounds. It begins by listing common binary ionic compounds and their formulas, noting they all end in -ide. It then discusses compounds containing complex ions, explaining the roman numerals in parentheses indicate the metal's valency. Finally, it lists the names of additional ionic compounds when given their formulas.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
22 views1 pageWriting & Naming Ionic Formulae
Uploaded by
AnonymousThe document provides information about writing ionic formulas for ionic compounds. It begins by listing common binary ionic compounds and their formulas, noting they all end in -ide. It then discusses compounds containing complex ions, explaining the roman numerals in parentheses indicate the metal's valency. Finally, it lists the names of additional ionic compounds when given their formulas.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
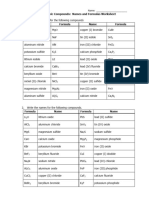
S3 Chemistry Ionic Bonding
Writing Ionic Formulae
Answer the following questions using the ionic formulae cards. Write the correct formula for the
ionic compound in the space provided. Elements & compounds not in the kit are in bold.
Easy (Binary Compounds)
Sodium chloride NaCl Calcium sulphide CaO
Potassium bromide KBr Aluminium oxide Al₂O₃
Sodium fluoride NaF Aluminium nitride AlN
Magnesium chloride MgCl₂ Magnesium nitride Mg₃N₂
Calcium bromide CaBr₂ Calcium phosphide Ca₃P₂
Aluminium fluoride AlF₃ Copper (II) chloride CuCl₂
Magnesium oxide MgO Vanadium (V) oxide V₂O₅
What do all of the binary compounds above have in common?
They all end in -ide.
Medium – Hard (contain complex ions)
Magnesium carbonate MgCO₃ Aluminium sulphate Al₂(SO₄)₃
Calcium sulphate CaSO₄ Aluminium nitrate Al(NO₃)₃
Potassium nitrate KNO₃ Magnesium nitrate Mg(NO₃)₂
Sodium carbonate Na₂CO₃ Copper (II) sulphate Cu SO₄
Ammonium (NH4)3PO4
Potassium sulphate K₂SO₄
phosphate
What do the roman numerals in brackets mean?
The valency of these transitional metals.
Now name the following compounds
KF Potassium fluoride KNO3 Potassium nitrate
CaCl2 Calcium chloride Ca(NO3)2 Calcium nitrate
AlF3 Aluminium fluoride Al2(CO3)3 Aluminium carbonate
CaO Calcium oxide MgSO4 Magnesium sulfate
Al2S3 Aluminium sulfide CuO Copper oxide
Na2O Sodium oxide Mg3N2 Magnesium nitride
MgBr2 Magnesium bromide Cu2O Copper oxide
NaBr Sodium bromide (NH4)2CO3 Ammonium carbonate
D.Gould, /conversion/tmp/scratch/483408943.docx, 21/05/2013.
You might also like
- Acids, Bases, & SaltsDocument30 pagesAcids, Bases, & Saltsmaghfirarizkar100% (1)
- Formula Writing - CambridgeDocument5 pagesFormula Writing - CambridgeQusai Saify100% (3)
- Chemical FormulaeDocument3 pagesChemical Formulaeparth chakre100% (2)
- Chapter 8: SaltsDocument23 pagesChapter 8: SaltsWong Wai LunNo ratings yet
- Metals and Non Metals - NotesDocument13 pagesMetals and Non Metals - NotesmittalshivamNo ratings yet
- Name of Atom Common Ionic ChargeDocument2 pagesName of Atom Common Ionic ChargeMichael Rey MendozaNo ratings yet
- Chemical Formula Writing Worksheet SolutionsDocument3 pagesChemical Formula Writing Worksheet SolutionsReid Pineda Creencia100% (1)
- Material Science Cheatsheet For Midterm (NEWEST)Document1 pageMaterial Science Cheatsheet For Midterm (NEWEST)DillNo ratings yet
- 4.1.2. Standard Solutions For Limit TestsDocument4 pages4.1.2. Standard Solutions For Limit TestsJjangyiNo ratings yet
- Interior Design StylesDocument16 pagesInterior Design StylesCric Sports BDNo ratings yet
- Hindustan Zinc Limited ReportDocument103 pagesHindustan Zinc Limited ReportManu Jain100% (3)
- Investment Chilean Mining Industry 2013 2021Document53 pagesInvestment Chilean Mining Industry 2013 2021AndreaRJCNo ratings yet
- Metalurgia Southern Peru Copper CorporationDocument27 pagesMetalurgia Southern Peru Copper CorporationYENY ROJAS HERRERANo ratings yet
- JIS Standards Conv2 Iso.Document1 pageJIS Standards Conv2 Iso.manbkkNo ratings yet
- Formula Kimia Dan Nama Bahan KimiaDocument2 pagesFormula Kimia Dan Nama Bahan Kimiahansm100% (5)
- 2.4.3 Chemical Formula and Naming Practice QuestionsDocument7 pages2.4.3 Chemical Formula and Naming Practice Questionsphat.vuongNo ratings yet
- Chemical Formulas The Boring SchoolDocument8 pagesChemical Formulas The Boring SchoolJigar BansalNo ratings yet
- Nomenclature WorksheetDocument2 pagesNomenclature WorksheetJoseph GagnonNo ratings yet
- Post 5.9. Ionic Compounds Practice - ANSWERSDocument3 pagesPost 5.9. Ionic Compounds Practice - ANSWERSAlan MartínNo ratings yet
- Nomenclature of Inorganic Compounds: Report SheetDocument3 pagesNomenclature of Inorganic Compounds: Report SheetAEsmilingNo ratings yet
- Chemical Formula WorksheetDocument4 pagesChemical Formula WorksheetMaria adeelNo ratings yet
- Chapter 1, Naming CompoundsDocument19 pagesChapter 1, Naming CompoundsKurdishNo ratings yet
- Dustin Kern - Writing Polyatomic Names and Formulas WorksheetDocument2 pagesDustin Kern - Writing Polyatomic Names and Formulas Worksheetapi-644456294No ratings yet
- Ionic Formulae Worksheet With AnswersDocument3 pagesIonic Formulae Worksheet With Answerssebastianjohnson1123No ratings yet
- 1.5B Solutions For Ionic Compounds, Extra ExercisesDocument1 page1.5B Solutions For Ionic Compounds, Extra ExercisesDaniel StandringNo ratings yet
- Lesson 1.0 - Questions 1. Write Standard Atomic Notation For H Ca F Li ODocument16 pagesLesson 1.0 - Questions 1. Write Standard Atomic Notation For H Ca F Li OThai NgoNo ratings yet
- Important Chemical Formulas The Boring SchoolDocument4 pagesImportant Chemical Formulas The Boring SchoolJigar BansalNo ratings yet
- Chemistry: Self Access Learning SheetDocument8 pagesChemistry: Self Access Learning SheetNooraini HusseinNo ratings yet
- Ionic Compounds Names and Formulas Worksheet AnswersDocument2 pagesIonic Compounds Names and Formulas Worksheet AnswersShayan UzzamanNo ratings yet
- Chm361 Chapter 5Document34 pagesChm361 Chapter 5syamimiafrinaNo ratings yet
- Exercise 14Document1 pageExercise 14clarissacastillo090904No ratings yet
- Inorganic Compounds: Chemical Name Chemical FormulaDocument6 pagesInorganic Compounds: Chemical Name Chemical FormulaFrendick LegaspiNo ratings yet
- Howtowritechemicalformulacomplete 110302230114 Phpapp01Document86 pagesHowtowritechemicalformulacomplete 110302230114 Phpapp01VladimirNo ratings yet
- Chemistry Chapter 1.exercise 1ADocument28 pagesChemistry Chapter 1.exercise 1AAsifNo ratings yet
- CIA4.1General Chemistry 1Document1 pageCIA4.1General Chemistry 1Marchelle MondezNo ratings yet
- Chm361-Chapter 5Document34 pagesChm361-Chapter 5atikah roshanNo ratings yet
- Nomenclature Exercise AnswersDocument3 pagesNomenclature Exercise AnswersAh TseNo ratings yet
- Review #3 NomenclatureDocument1 pageReview #3 NomenclatureCassandra MachadoNo ratings yet
- Chemical Formula Binary Ionic CompoundsDocument2 pagesChemical Formula Binary Ionic CompoundsRamisNo ratings yet
- Baso Agno H S Cao H Co MG (Po) K Cro NaiDocument2 pagesBaso Agno H S Cao H Co MG (Po) K Cro NaiAmy BalicagNo ratings yet
- Jordan Paddock - Writing Polyatomic Names and Formulas WorksheetDocument1 pageJordan Paddock - Writing Polyatomic Names and Formulas Worksheetapi-675312232No ratings yet
- Chemical NomenclatureDocument7 pagesChemical NomenclatureQuỳnh NgânNo ratings yet
- Ionic Compounds Worksheet-IiiDocument1 pageIonic Compounds Worksheet-IiitylerNo ratings yet
- Syed ArsalanDocument1 pageSyed ArsalanShahzaib MughalNo ratings yet
- Symbol - Equations - Homework RMDocument2 pagesSymbol - Equations - Homework RMayaanrayhaanNo ratings yet
- Test 1 Formula of IonsDocument6 pagesTest 1 Formula of IonsSEAW FUI MINGNo ratings yet
- Formulas of Compounds Polyatomics KEYDocument2 pagesFormulas of Compounds Polyatomics KEYJewel Emerald C. CudiamatNo ratings yet
- Colour of IonsDocument2 pagesColour of Ionsbooksale SiNo ratings yet
- Chemical Formula Writing WorksheetDocument5 pagesChemical Formula Writing WorksheetÂziz ShuvoNo ratings yet
- DANH PHÁP HÓA HỌC MỚIDocument6 pagesDANH PHÁP HÓA HỌC MỚILe Huy TranNo ratings yet
- Nomenclature ExerciseDocument5 pagesNomenclature ExerciseAh TseNo ratings yet
- List of Important Metals and Their Ores With Chemical Formulas PDFDocument2 pagesList of Important Metals and Their Ores With Chemical Formulas PDFAudibleNo ratings yet
- Homework 4.2 Naming and Writing of Chemical FomulaDocument2 pagesHomework 4.2 Naming and Writing of Chemical FomulaRenzmario LumabiNo ratings yet
- 1.5A Ionic Compounds, Extra ExercisesDocument2 pages1.5A Ionic Compounds, Extra ExercisesDaniel StandringNo ratings yet
- Chapter 5 Coordination CompoundDocument36 pagesChapter 5 Coordination Compoundammar zakariaNo ratings yet
- Ionic Compound Naming KeyDocument1 pageIonic Compound Naming Keynisrina amaliaNo ratings yet
- Compound Name Molecular Formula Compound Name Molecular FormulaDocument4 pagesCompound Name Molecular Formula Compound Name Molecular Formulamohamed ahmedNo ratings yet
- Ionic Compound Formula Writing WorksheetDocument2 pagesIonic Compound Formula Writing WorksheetJackson LtorishaNo ratings yet
- Heat Cao S Ho Ca Oh Aq: Hol H G OgDocument12 pagesHeat Cao S Ho Ca Oh Aq: Hol H G OgValyanaNo ratings yet
- Nomenclature Assignment Part 1Document4 pagesNomenclature Assignment Part 1marNo ratings yet
- Namingpacketanswers 3Document14 pagesNamingpacketanswers 3Supremo DelagerNo ratings yet
- Green and Yellow Doodle Science Project Cover A4 DocumentDocument15 pagesGreen and Yellow Doodle Science Project Cover A4 DocumentDhiren vollalaNo ratings yet
- Ella ScienceDocument2 pagesElla ScienceLorna Ojarliza AchaNo ratings yet
- Naming - Compounds - Mixed With AnswersDocument4 pagesNaming - Compounds - Mixed With AnswerslinaNo ratings yet
- Chemical Formula Worksheet: ST NDDocument2 pagesChemical Formula Worksheet: ST NDsherlyn may lolNo ratings yet
- Booklet On Acid and Base and Redox MSDocument43 pagesBooklet On Acid and Base and Redox MShalahossam8899No ratings yet
- Microsoft Word - Ionic-CovalentNameRace1Document2 pagesMicrosoft Word - Ionic-CovalentNameRace1cen BsitNo ratings yet
- Describe and Explain How Lithium Forms An Ionic CompoundDocument1 pageDescribe and Explain How Lithium Forms An Ionic CompoundAnonymousNo ratings yet
- Drawing Ions: S3 IGCSE Chemistry Topic 1: Ionic BondingDocument1 pageDrawing Ions: S3 IGCSE Chemistry Topic 1: Ionic BondingAnonymousNo ratings yet
- Investigating The Effect of Concentration On The Rate of ReactionDocument4 pagesInvestigating The Effect of Concentration On The Rate of ReactionAnonymousNo ratings yet
- Naming Ionic CompundsDocument1 pageNaming Ionic CompundsAnonymousNo ratings yet
- Helen Keller A Woman To RememberDocument2 pagesHelen Keller A Woman To RememberAnonymousNo ratings yet
- Gangsta Granny: By: Arianna ToledoDocument5 pagesGangsta Granny: By: Arianna ToledoAnonymousNo ratings yet
- Oceania Thought Police Department Sworn Confession: Winston SDocument2 pagesOceania Thought Police Department Sworn Confession: Winston SAnonymousNo ratings yet
- Helen Keller: By: Arianna ToledoDocument1 pageHelen Keller: By: Arianna ToledoAnonymousNo ratings yet
- Russia, 1905-41 - Terror + PurgesDocument11 pagesRussia, 1905-41 - Terror + PurgesAnonymousNo ratings yet
- AMC Mining Brochure (A4 LR)Document2 pagesAMC Mining Brochure (A4 LR)Bandung WestNo ratings yet
- Cru Breakfast 2020 Session 1 ChinaDocument26 pagesCru Breakfast 2020 Session 1 ChinaDebarkaChakrabortyNo ratings yet
- Night School 18 Session 2Document67 pagesNight School 18 Session 2Luis CortesNo ratings yet
- Chemistry Class 9 21 Feb 15Document8 pagesChemistry Class 9 21 Feb 15Muhammad ObaidullahNo ratings yet
- Fischer Tiplovi Vijci Za Porobeton Aircrete - (V5) - 07062012Document12 pagesFischer Tiplovi Vijci Za Porobeton Aircrete - (V5) - 07062012namkvalNo ratings yet
- Cambridge IGCSE: Chemistry 0620/23Document16 pagesCambridge IGCSE: Chemistry 0620/23mostafa barakatNo ratings yet
- Effect of Cryogenic Treatment On CompositesDocument14 pagesEffect of Cryogenic Treatment On CompositesGauthamNo ratings yet
- Art PDFDocument37 pagesArt PDFLuminita AndronicNo ratings yet
- Barcelona ScriptDocument35 pagesBarcelona ScriptSerious ComedianNo ratings yet
- Unit 3 Study GuideDocument15 pagesUnit 3 Study GuideYouree Choi0% (1)
- Construction Drawing: All Installation Detail That Not Stated in This Drawing Are Complies With Specifications DetailDocument1 pageConstruction Drawing: All Installation Detail That Not Stated in This Drawing Are Complies With Specifications DetailrajavelNo ratings yet
- 2 Periodic Table PRACTICE TestDocument6 pages2 Periodic Table PRACTICE TestAlNo ratings yet
- Catalyst Deactivation - Is It Predictable What To Do PDFDocument14 pagesCatalyst Deactivation - Is It Predictable What To Do PDFBamrung SungnoenNo ratings yet
- SB9 AnsDocument66 pagesSB9 AnsCarl Masuret100% (1)
- Document PDFDocument8 pagesDocument PDFDebray Rodriguez EsquivelNo ratings yet
- Chemistry Pahang JUJ 2008 (Edu - Joshuatly.com)Document55 pagesChemistry Pahang JUJ 2008 (Edu - Joshuatly.com)Apple KWNo ratings yet
- Chemical Reaction ActivityDocument2 pagesChemical Reaction ActivityGilynneNo ratings yet
- Chemistry Exam 1Document6 pagesChemistry Exam 1jshalda1No ratings yet
- Study of Bauschinger Effect in SpringDocument95 pagesStudy of Bauschinger Effect in Springmail_sambhuNo ratings yet
- CastingDocument65 pagesCastingsamurai7_77No ratings yet
- Yss Tool Steels BDocument77 pagesYss Tool Steels BneramjanNo ratings yet