Professional Documents
Culture Documents
Nomenclature Exercise Answers
Uploaded by
Ah Tse0 ratings0% found this document useful (0 votes)
13 views3 pagesOriginal Title
nomenclature exercise answers
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
13 views3 pagesNomenclature Exercise Answers
Uploaded by
Ah TseCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 3
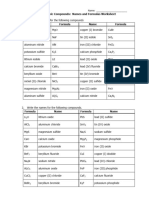
SNC2D - Grade 10 Academic Science
Chemistry Triathlon - Molecular & Ionic Compounds and Acids & Bases
For the mixed problems, be aware of what rules you are using. Use the naming/formula flow chart to
help guide you!
Provide the formula or IUPAC & Classical names where possible.
Calcium nitride Ca3N2
Boron mononitride BN
Tellurium dichloride TeCl2
Strontium sulfide SrS
Cobalt (II) sulfide CoS
Nickel (III) sulfide Ni2S3
Chromium (III) chloride CrCl3
Magnesium oxide MgO
Nitrogen monoxide NO
Diphosphorus pentoxide P2O5
Iron(III) nitride FeN
Gallium chloride GaCl3
tetraphosphorus pentoxide P4O5
Rubidium chloride RbCl
Mercury (II) chloride HgCl2
Selenium dibromide SeBr2
Nitrogen tribromide NBr3
Aluminum sulphide Al2S3
Aluminum oxide Al2O3
Iron (III) oxide, ferric oxide Fe2O3
Ammonium phosphide (NH4)3P
Hydrogen sulfide H2S
SNC2D - Grade 10 Academic Science
Potassium chloride KCl
Hydrogen bromide HBr
Fluorine F2 (aq)
Carbon dioxide CO2
Diarsenic trisulfide As2S3
Hydrogen peroxide H2O2
Oxygen gas O2
Sodium hydroxide NaOH
carbon disulphide CS2
lithium sulphide Li2S
titanium (IV) chloride TiCl4
Hydrogen chloride HCl
copper (II) hydroxide Cu(OH)2
magnesium nitrate Mg(NO3)2
Lead(IV) chlorate Pb(ClO3)4
Hydrogen Phosphate H3PO4
Ammonium oxide (NH4)2O
Magnesium hydroxide Mg(OH)2
Copper (II) chloride CuCl2
Bromine Br2 (g)
Hydrogen Iodide (Hydroiodic acid) HI (aq)
Dinitrogen pentoxide N2O5
Copper (II) bromide CuBr2
Diphosphorus pentoxide P2O5
Nickel (II) phosphide Ni3P2
SNC2D - Grade 10 Academic Science
Hydrogen Nitrate HNO3 (aq)
Calcium phosphide Ca3P2
ammonia NH3
Ammonium fluoride NH4F
Iron (II) chloride FeCl2
nitrogen monoxide NO
magnesium cyanide Mg(CN)2
Ammonium hydroxide NH4OH
calcium carbonate CaCO3
Dinitogen tetroxide N2O4
sodium nitride Na3N
Phosphorus pentaiodide PI5
Aluminum hydroxide Al(OH)3
You might also like
- Nomenclature WorksheetDocument5 pagesNomenclature WorksheetJapphetNo ratings yet
- Chemical Formula Worksheet: Write Cation-Anion CombosDocument4 pagesChemical Formula Worksheet: Write Cation-Anion Combosprabhu4321100% (1)
- Nomenclature WorksheetDocument2 pagesNomenclature WorksheetJoseph GagnonNo ratings yet
- Nomenclature Assignment Part 1Document4 pagesNomenclature Assignment Part 1marNo ratings yet
- Lesson 1.0 - Questions 1. Write Standard Atomic Notation For H Ca F Li ODocument16 pagesLesson 1.0 - Questions 1. Write Standard Atomic Notation For H Ca F Li OThai NgoNo ratings yet
- Common Polyatomic IonsDocument1 pageCommon Polyatomic IonsRoddyNo ratings yet
- Post 5.9. Ionic Compounds Practice - ANSWERSDocument3 pagesPost 5.9. Ionic Compounds Practice - ANSWERSAlan MartínNo ratings yet
- Dustin Kern - Writing Polyatomic Names and Formulas WorksheetDocument2 pagesDustin Kern - Writing Polyatomic Names and Formulas Worksheetapi-644456294No ratings yet
- Inorganic Compounds: Chemical Name Chemical FormulaDocument6 pagesInorganic Compounds: Chemical Name Chemical FormulaFrendick LegaspiNo ratings yet
- Fall 2021/STEM1-Chemistry/Worksheet 5/chapter 2.6-2.7/Dr. LingDocument3 pagesFall 2021/STEM1-Chemistry/Worksheet 5/chapter 2.6-2.7/Dr. LingMohamed alharthiNo ratings yet
- 2 WeekDocument2 pages2 WeekAna Carballo TrabazoNo ratings yet
- 2.4.3 Chemical Formula and Naming Practice QuestionsDocument7 pages2.4.3 Chemical Formula and Naming Practice Questionsphat.vuongNo ratings yet
- Chemical FormulaeDocument3 pagesChemical Formulaeparth chakre100% (2)
- What Is The Systematic Name of The Following Compound (Solved)Document7 pagesWhat Is The Systematic Name of The Following Compound (Solved)Debayanbasu.juNo ratings yet
- Nomenclature ExerciseDocument5 pagesNomenclature ExerciseAh TseNo ratings yet
- Formulae and Naming RevisionDocument1 pageFormulae and Naming RevisionDivya SonkhlaNo ratings yet
- List of Cation and AnionDocument2 pagesList of Cation and Anionnewtonenergy17No ratings yet
- Nomenclature of Inorganic Compounds: Report SheetDocument3 pagesNomenclature of Inorganic Compounds: Report SheetAEsmilingNo ratings yet
- Common Anions and CationsDocument4 pagesCommon Anions and CationsaosobNo ratings yet
- List of Common IonsDocument3 pagesList of Common IonsangelonicoNo ratings yet
- Homework 4.2 Naming and Writing of Chemical FomulaDocument2 pagesHomework 4.2 Naming and Writing of Chemical FomulaRenzmario LumabiNo ratings yet
- DANH PHÁP HÓA HỌC MỚIDocument6 pagesDANH PHÁP HÓA HỌC MỚILe Huy TranNo ratings yet
- Chapter - 7 Correction Naming CompoundsDocument2 pagesChapter - 7 Correction Naming CompoundsMurad IsayevNo ratings yet
- Formula Kimia Dan Nama Bahan KimiaDocument2 pagesFormula Kimia Dan Nama Bahan Kimiahansm100% (5)
- Common Polyatomic Ions and Chemical CompoundsDocument4 pagesCommon Polyatomic Ions and Chemical CompoundsBhel San Pedro MarzanNo ratings yet
- Symbol - Equations - Homework RMDocument2 pagesSymbol - Equations - Homework RMayaanrayhaanNo ratings yet
- Ionic Compounds Names and Formulas Worksheet AnswersDocument2 pagesIonic Compounds Names and Formulas Worksheet AnswersShayan UzzamanNo ratings yet
- Activity5 ChemicalformulasDocument2 pagesActivity5 ChemicalformulasJohn Hayden Dela CruzNo ratings yet
- Baso Agno H S Cao H Co MG (Po) K Cro NaiDocument2 pagesBaso Agno H S Cao H Co MG (Po) K Cro NaiAmy BalicagNo ratings yet
- Naming Common Chemical CompoundsDocument7 pagesNaming Common Chemical CompoundsSnorlax Magno100% (1)
- ions ref sheetDocument2 pagesions ref sheetmoyston.jade2No ratings yet
- JRS Tutorials: Chemistry IITDocument58 pagesJRS Tutorials: Chemistry IITtusharr11.mobNo ratings yet
- ionicChargesChart PDFDocument1 pageionicChargesChart PDFronit675No ratings yet
- Chemical Formula Binary Ionic CompoundsDocument2 pagesChemical Formula Binary Ionic CompoundsRamisNo ratings yet
- Chemical CompoundsDocument1 pageChemical CompoundsIvy Michelle HabagatNo ratings yet
- Chemical Formula Writing Worksheet PDFDocument4 pagesChemical Formula Writing Worksheet PDFkezia0% (1)
- List of IonsDocument1 pageList of IonsIsha Nathalie GalimbaNo ratings yet
- Name and write formulas for common compoundsDocument1 pageName and write formulas for common compoundsMarchelle MondezNo ratings yet
- Chemistry - WS Combo #1Document2 pagesChemistry - WS Combo #1thomasnakyra623No ratings yet
- Nomenclature Worksheet NDocument2 pagesNomenclature Worksheet NVictor GarciaNo ratings yet
- Cation Positive Ion Charge ChartDocument6 pagesCation Positive Ion Charge ChartSEAW FUI MINGNo ratings yet
- Problem Set No. 1Document3 pagesProblem Set No. 1Ej Ferrer100% (1)
- Common Ions TableDocument1 pageCommon Ions TableAbu KamiliaNo ratings yet
- 50 Geology Minerals: Alphabetically WrittenDocument2 pages50 Geology Minerals: Alphabetically Writtenorjiugo.victorNo ratings yet
- Screenshot 2022-12-05 at 7.29.25 AMDocument1 pageScreenshot 2022-12-05 at 7.29.25 AMfeiNo ratings yet
- Review #3 NomenclatureDocument1 pageReview #3 NomenclatureCassandra MachadoNo ratings yet
- Cations: Al Aluminium Fe Iron (III) CR Chromium (III)Document2 pagesCations: Al Aluminium Fe Iron (III) CR Chromium (III)NPNo ratings yet
- More Extra Nomenclature Practice - KEYDocument10 pagesMore Extra Nomenclature Practice - KEYelitzelmartinez21No ratings yet
- NAMING AND WRITING FORMULAS FOR COMPOUNDSDocument2 pagesNAMING AND WRITING FORMULAS FOR COMPOUNDSMichael Rey MendozaNo ratings yet
- Write formulas, names, and balance equationsDocument2 pagesWrite formulas, names, and balance equationsAna LuisaNo ratings yet
- Hydrogencarbonate Mercury (II) Hydrogen Sulfate Mercury (I) Hydrogen Sulfite OrthoborateDocument1 pageHydrogencarbonate Mercury (II) Hydrogen Sulfate Mercury (I) Hydrogen Sulfite OrthoborateVanessa RuizNo ratings yet
- Ionic Compounds Worksheet-IiiDocument1 pageIonic Compounds Worksheet-IiitylerNo ratings yet
- Ion NamesDocument3 pagesIon NamesSatyamNo ratings yet
- Heat Cao S Ho Ca Oh Aq: Hol H G OgDocument12 pagesHeat Cao S Ho Ca Oh Aq: Hol H G OgValyanaNo ratings yet
- List of Ions: I. CationsDocument4 pagesList of Ions: I. CationsJamille GamboaNo ratings yet
- Common Ions and Their ChargesDocument2 pagesCommon Ions and Their ChargesTristanEvangelistaNo ratings yet
- Chem 1 List of IonsDocument2 pagesChem 1 List of IonsJean Angelove SantosNo ratings yet
- Handbook of Reagents for Organic Synthesis: Reagents for Heteroarene FunctionalizationFrom EverandHandbook of Reagents for Organic Synthesis: Reagents for Heteroarene FunctionalizationNo ratings yet
- The Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyFrom EverandThe Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyNo ratings yet
- Analysis of the New Metals: Titanium, Zirconium, Hafnium, Niobium, Tantalum, Tungsten and Their AlloysFrom EverandAnalysis of the New Metals: Titanium, Zirconium, Hafnium, Niobium, Tantalum, Tungsten and Their AlloysNo ratings yet
- Handbook of Preparative Inorganic Chemistry V2From EverandHandbook of Preparative Inorganic Chemistry V2Georg BrauerNo ratings yet
- "Gray Iron" "Ductile Iron": Alloy Cast IronsDocument2 pages"Gray Iron" "Ductile Iron": Alloy Cast Ironsandrian hermanNo ratings yet
- Is: 2062-1981 Mild SteelDocument2 pagesIs: 2062-1981 Mild Steelsuresh kumarNo ratings yet
- Atomic Structure Part 6Document38 pagesAtomic Structure Part 6xenaNo ratings yet
- UTP Bestseller PDFDocument28 pagesUTP Bestseller PDFdaha333No ratings yet
- ME1107 Welding AllDocument130 pagesME1107 Welding AllRizuanul Arefin EmonNo ratings yet
- Mineral and Energy Resources GuideDocument4 pagesMineral and Energy Resources GuideJohn WickNo ratings yet
- Tube Forming Processes A Comprehensive Guide (Greg G. Miller)Document378 pagesTube Forming Processes A Comprehensive Guide (Greg G. Miller)An NguyenNo ratings yet
- Batterycontacts DuracellDocument9 pagesBatterycontacts DuracellwilliaqNo ratings yet
- Electroplating and Metal Refining Worksheet Solutions Y9dbujDocument3 pagesElectroplating and Metal Refining Worksheet Solutions Y9dbujVaida MatulevičiūtėNo ratings yet
- Periodic Table of The ElementsDocument1 pagePeriodic Table of The Elementskaren listNo ratings yet
- Employee Satisfaction: Hindustan Zinc Smelter DebariDocument65 pagesEmployee Satisfaction: Hindustan Zinc Smelter Debarirjjain07No ratings yet
- Occurrence and Extraction of Metals: Module - 6Document15 pagesOccurrence and Extraction of Metals: Module - 6Manish kumarNo ratings yet
- Design and Manufacture of A Hydraulic ExDocument10 pagesDesign and Manufacture of A Hydraulic ExRamdas JoshiNo ratings yet
- Astm A350Document8 pagesAstm A350nse mcx100% (1)
- Duralumin Poles Are Preferred To Those Made of FiberglassDocument1 pageDuralumin Poles Are Preferred To Those Made of FiberglassAnca TruţaNo ratings yet
- Material Selection For Chemical Process Equipment: Engr. Sandino Michael Angelo G. Aguilar, Che Subject InstructorDocument26 pagesMaterial Selection For Chemical Process Equipment: Engr. Sandino Michael Angelo G. Aguilar, Che Subject InstructorGautam VadnereNo ratings yet
- Testing Silver Ores SimplifiedDocument130 pagesTesting Silver Ores Simplifiedkevin testNo ratings yet
- Milling Process, Defects, EquipmentDocument6 pagesMilling Process, Defects, Equipmentdeuvyn bautistaNo ratings yet
- A 982 - 02 Perfiles de TurbinaDocument4 pagesA 982 - 02 Perfiles de Turbinaalucard375No ratings yet
- Caution - Turbine 2 - 13961340 - Pt. SKS Listrik Kalimantan - Turbine Lube Oil - 1Document2 pagesCaution - Turbine 2 - 13961340 - Pt. SKS Listrik Kalimantan - Turbine Lube Oil - 1dhavit wijayantoNo ratings yet
- Metal Notes PlumbingDocument8 pagesMetal Notes PlumbingPatrick MachariaNo ratings yet
- A Historical Perspective of Synthetic Ceramic and Traditional Feldspathic PorcelainDocument7 pagesA Historical Perspective of Synthetic Ceramic and Traditional Feldspathic PorcelainCarissaNo ratings yet
- NBIMS-US V3 2.4.4.12 OmniClass Table 41 MaterialsDocument22 pagesNBIMS-US V3 2.4.4.12 OmniClass Table 41 Materialsmahmoud mokhtarNo ratings yet
- Aird 2021Document15 pagesAird 2021alanNo ratings yet
- Chemical Engineering Material Iron-Carbon-DiagramsDocument12 pagesChemical Engineering Material Iron-Carbon-Diagramsaditya_lomteNo ratings yet
- Material Hardware July 201Document30 pagesMaterial Hardware July 201SuryaNo ratings yet
- Brochure SunflagDocument12 pagesBrochure SunflagkunalNo ratings yet
- Yoga and its Elements Project FileDocument18 pagesYoga and its Elements Project FileAdarsh SrivastavaNo ratings yet
- A Textbook On HydrometallurgyDocument754 pagesA Textbook On HydrometallurgyWaode Febry Nurfadhila100% (1)