Professional Documents
Culture Documents
Chemistry - WS Combo #1
Uploaded by
thomasnakyra6230 ratings0% found this document useful (0 votes)
3 views2 pagesOriginal Title
Chemistry- WS Combo #1
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
3 views2 pagesChemistry - WS Combo #1
Uploaded by
thomasnakyra623Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
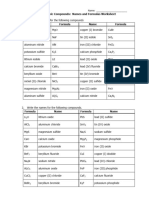
Chemistry
Goal 1 Chem. Obj. 1.2.4
Naming Ionic Compounds Name: na’kyra thomas-boone
Date: 2/9/24 Per: 4
Directions: Identify each of the following as binary ionic (B), binary ionic with a transition metal (BT), ionic with a
polyatomic ion (P) or ionic with transition metal and polyatomic ion (TP). Then write the name for each
compound.
Type of
Chemical Formula Chemical Name
Compound
1. MgCl2 B Magnesium dichloride
2. PbCl2 B Lead chloride
3. Sn(OH)2 P Tin(II) hydroxide
4. CoSO4 BT cobalt sulfate
5. KBr B potassium bromide
6. SnS2 B tin sulfide
7. PbBr4 B lead (IV) Bromide
8. Fe2(SO4)3 TP iron (III) sulfate
9. Li3PO4 B lithium phosphate
10. AlF3 B aluminum fluoride
11. Cu(NO3)2 TP copper(II) nitrate
12. FeN BT iron (III) nitride
13. KNO2 B potassium nitrite
14. Li3N B lithium nitride
15. Sr(ClO3)2 P strontium chlorate
16. Ba3N2 B barium nitride
17. Na2SO3 B sodium sulfite
18. Ba(NO3)2 P barium nitrate
19. Cu3P BT copper phosphide
20. MgSO4 B magnesium sulfate
Nonmetal Binary Covalent Compounds
Complete the chart by providing either the correct formula or name. The first row has been filled in as an
example.
.
Name Formula Name Formula
nitrogen dioxide NO2 Phosphorus trichloride PCl3 ____
Sulfur tetrachloride SCl4 sulfur hexafluoride SF6
hydrogen monochloride HIC hydrogen sulfide H2S(g)
phosphorus triiodide PI3 disulfur dichloride S2CI2
dinitrogen tetroxide N2O4 dinitrogen trioxide N2O3
antimony trichloride SbCl3 Antimony pentachloride SbCl5
silicon dioxide SiO carbon monoxide Co
trioxosilicate SiO3 carbon dioxide co2
phosphorus trihydride PH3 Nitric oxide NO
You might also like
- Coordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972From EverandCoordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972A. B. P. LeverNo ratings yet
- Common Polyatomic IonsDocument1 pageCommon Polyatomic IonsRoddyNo ratings yet
- Lesson 1.0 - Questions 1. Write Standard Atomic Notation For H Ca F Li ODocument16 pagesLesson 1.0 - Questions 1. Write Standard Atomic Notation For H Ca F Li OThai NgoNo ratings yet
- IB Chemistry-Revision On Naming Ionic CompoundsDocument3 pagesIB Chemistry-Revision On Naming Ionic CompoundsRania ShabanNo ratings yet
- Chem (LAS)Document2 pagesChem (LAS)mhyrela roncedNo ratings yet
- Name of Atom Common Ionic ChargeDocument2 pagesName of Atom Common Ionic ChargeMichael Rey MendozaNo ratings yet
- Nomenclature Review AssignmentDocument8 pagesNomenclature Review AssignmentTish BarnesNo ratings yet
- Pcqa111 - Assignment For Nomenclature and Formula WritingDocument1 pagePcqa111 - Assignment For Nomenclature and Formula WritingRusselle Kate AlvaradoNo ratings yet
- Chapter 1, Naming CompoundsDocument19 pagesChapter 1, Naming CompoundsKurdishNo ratings yet
- Ion Old Iupac Ion Old Name IupacDocument1 pageIon Old Iupac Ion Old Name IupacjenduekieNo ratings yet
- Q1Document1 pageQ1Jant Erbert GarbosoNo ratings yet
- GenChem Nomenclature Updated PDFDocument2 pagesGenChem Nomenclature Updated PDFCamille AquinoNo ratings yet
- Nomenclature Worksheet NDocument2 pagesNomenclature Worksheet NVictor GarciaNo ratings yet
- Laboratory Periodic Table: Gsci1103L-General Chemistry 1 LabDocument6 pagesLaboratory Periodic Table: Gsci1103L-General Chemistry 1 LabAndrea AurielleNo ratings yet
- Nomenclature Exercise AnswersDocument3 pagesNomenclature Exercise AnswersAh TseNo ratings yet
- Chemlec 10.2Document2 pagesChemlec 10.2Ana LuisaNo ratings yet
- Ion Old Iupac Ion Old Name IupacDocument1 pageIon Old Iupac Ion Old Name IupacchelliNo ratings yet
- Fall 2021/STEM1-Chemistry/Worksheet 5/chapter 2.6-2.7/Dr. LingDocument3 pagesFall 2021/STEM1-Chemistry/Worksheet 5/chapter 2.6-2.7/Dr. LingMohamed alharthiNo ratings yet
- Problem Set No. 1Document3 pagesProblem Set No. 1Ej Ferrer100% (1)
- Unit 4 Naming Amp Types Naming Polyatomic Compounds Worksheet 2 Page 1 AnswersDocument2 pagesUnit 4 Naming Amp Types Naming Polyatomic Compounds Worksheet 2 Page 1 AnswersdiahemaNo ratings yet
- Dustin Kern - Writing Polyatomic Names and Formulas WorksheetDocument2 pagesDustin Kern - Writing Polyatomic Names and Formulas Worksheetapi-644456294No ratings yet
- Positive Monoatomic Ions Negative Polyatomic IonsDocument1 pagePositive Monoatomic Ions Negative Polyatomic IonsGeah LeeNo ratings yet
- Common Ions Table PDFDocument1 pageCommon Ions Table PDFAnnabelleNo ratings yet
- Common Ions and Their ChargesDocument2 pagesCommon Ions and Their ChargesTristanEvangelistaNo ratings yet
- JRS Tutorials: Chemistry IITDocument58 pagesJRS Tutorials: Chemistry IITtusharr11.mobNo ratings yet
- Chemistry ReviewerDocument4 pagesChemistry ReviewerBhel San Pedro MarzanNo ratings yet
- List of Ions: I. CationsDocument4 pagesList of Ions: I. CationsJamille GamboaNo ratings yet
- Symbols and Charges-Monoatomic IonsDocument20 pagesSymbols and Charges-Monoatomic Ionsjon_kasilagNo ratings yet
- Nomenclature of Inorganic Compounds: Report SheetDocument3 pagesNomenclature of Inorganic Compounds: Report SheetAEsmilingNo ratings yet
- Ionic Nomenclature PracticeDocument5 pagesIonic Nomenclature PracticevanammanNo ratings yet
- Simple Binary Ionic Compounds: Nomenclature Worksheet 2Document4 pagesSimple Binary Ionic Compounds: Nomenclature Worksheet 2NameNo ratings yet
- Nomenclature WorksheetDocument2 pagesNomenclature WorksheetJoseph GagnonNo ratings yet
- Ion and AnionDocument1 pageIon and AnionciciNo ratings yet
- General Chemistry 2 Module 3Document6 pagesGeneral Chemistry 2 Module 3Jason Vinluan CarinanNo ratings yet
- Inorganic Nomenclature Rules, Examples and Practice Problems Bansal PatternDocument7 pagesInorganic Nomenclature Rules, Examples and Practice Problems Bansal PatternKumarNo ratings yet
- Ws Naming Compounds 9-11-08Document2 pagesWs Naming Compounds 9-11-08Yahra Aquino100% (1)
- OXIDATIONDocument1 pageOXIDATIONAdrian SwiftNo ratings yet
- 5.9 Polyatomic CompoundsDocument3 pages5.9 Polyatomic Compoundsmichael.delaney8541No ratings yet
- Nomenclature WorksheetDocument5 pagesNomenclature WorksheetJapphetNo ratings yet
- Common IonsDocument3 pagesCommon IonsabdallaaNo ratings yet
- Naming Review Practice Answer KeyDocument1 pageNaming Review Practice Answer Keyapi-376281962No ratings yet
- Inorganic Compounds: Chemical Name Chemical FormulaDocument6 pagesInorganic Compounds: Chemical Name Chemical FormulaFrendick LegaspiNo ratings yet
- Post 5.9. Ionic Compounds Practice - ANSWERSDocument3 pagesPost 5.9. Ionic Compounds Practice - ANSWERSAlan MartínNo ratings yet
- What Is The Systematic Name of The Following Compound (Solved)Document7 pagesWhat Is The Systematic Name of The Following Compound (Solved)Debayanbasu.juNo ratings yet
- More Extra Nomenclature Practice - KEYDocument10 pagesMore Extra Nomenclature Practice - KEYelitzelmartinez21No ratings yet
- GC1 Problem Set 1 Naming - GROUP 7Document3 pagesGC1 Problem Set 1 Naming - GROUP 7Louierose Joy CopreNo ratings yet
- Symbol and Charges For Monoatomic and Polyatomic Ions, Oxidation Number, and Acid NamesDocument3 pagesSymbol and Charges For Monoatomic and Polyatomic Ions, Oxidation Number, and Acid NamesKelvin Mark KaabayNo ratings yet
- Nomenclature Assignment Part 1Document4 pagesNomenclature Assignment Part 1marNo ratings yet
- Namingpacketanswers 3Document14 pagesNamingpacketanswers 3Supremo DelagerNo ratings yet
- 2 WeekDocument2 pages2 WeekAna Carballo TrabazoNo ratings yet
- Eleazar - Quiz#3Document2 pagesEleazar - Quiz#3ゆかりNo ratings yet
- DANH PHÁP HÓA HỌC MỚIDocument6 pagesDANH PHÁP HÓA HỌC MỚILe Huy TranNo ratings yet
- HomeworkDocument1 pageHomeworkkodaNo ratings yet
- Calventas Lab ReportDocument5 pagesCalventas Lab ReportGodwayneNo ratings yet
- 5-Ternary Ionic CompoundsDocument1 page5-Ternary Ionic CompoundsmargaritaisabellechamNo ratings yet
- Formula Writing - CambridgeDocument5 pagesFormula Writing - CambridgeQusai Saify100% (3)
- List of Cations and AnionsDocument2 pagesList of Cations and AnionsArvin MagtotoNo ratings yet
- Exercise 1a ChemistryDocument10 pagesExercise 1a Chemistryapi-533545229No ratings yet
- Ionic Compounds Names and Formulas Worksheet AnswersDocument2 pagesIonic Compounds Names and Formulas Worksheet AnswersShayan UzzamanNo ratings yet
- Honors Chemistry WKSHT Names and Formulas V and ANSWERSDocument2 pagesHonors Chemistry WKSHT Names and Formulas V and ANSWERSkijijisellerNo ratings yet
- C2 The Periodic Table Student Book AnswersDocument7 pagesC2 The Periodic Table Student Book AnswersjoeNo ratings yet
- Magnetism SC L1-3Document24 pagesMagnetism SC L1-3Abhishek tiwariNo ratings yet
- Indiana University Doctoral DissertationsDocument4 pagesIndiana University Doctoral DissertationsFindSomeoneToWriteMyPaperLittleRock100% (1)
- Q4-Worksheet - Week 8Document8 pagesQ4-Worksheet - Week 8Gian EvangelistaNo ratings yet
- Basics of The Iron and Steel IndustryDocument10 pagesBasics of The Iron and Steel Industryabdulaziz alharaziNo ratings yet
- Chapter-1 Ishida2011Document79 pagesChapter-1 Ishida2011sasidharkanthetiNo ratings yet
- Disadvantages of Hard Water Boiler TroublesDocument6 pagesDisadvantages of Hard Water Boiler Troubleskhushboo goyalNo ratings yet
- Self Assembled Nanoreactors PDFDocument46 pagesSelf Assembled Nanoreactors PDFandra mNo ratings yet
- Fly Ash Based Light Weight Geopolymer ConcreteDocument7 pagesFly Ash Based Light Weight Geopolymer Concretegowtham svNo ratings yet
- 12.2.2 Preparation of AlkenesDocument35 pages12.2.2 Preparation of AlkenesMUHAMMAD LUQMAN HAKIMI MOHD ZAMRINo ratings yet
- INDIAN PHARMACOPOEIA 2010 Volume 2 PDFDocument1,022 pagesINDIAN PHARMACOPOEIA 2010 Volume 2 PDFPradipta Mondal89% (9)
- Solution & Colligative Properties - PLPNDocument69 pagesSolution & Colligative Properties - PLPNvihaann2006No ratings yet
- Ion Channels PDFDocument7 pagesIon Channels PDFHyunji KimNo ratings yet
- Manufacturing Process of Sodium CarboxymethylcelluloseDocument2 pagesManufacturing Process of Sodium CarboxymethylcelluloserezaNo ratings yet
- Module 6 Gram Staining PreLabDocument24 pagesModule 6 Gram Staining PreLabcloudNo ratings yet
- Hydrogen (Inorganic Chemistry) Class 11thDocument4 pagesHydrogen (Inorganic Chemistry) Class 11thAnirudha ThakurNo ratings yet
- Initial PH (I-Ph) - Value of Petroleum Products: Standard Test Method ForDocument5 pagesInitial PH (I-Ph) - Value of Petroleum Products: Standard Test Method ForasmaNo ratings yet
- Stability of The Solid: Sodium Hypochlorite Is ADocument2 pagesStability of The Solid: Sodium Hypochlorite Is AVinod NairNo ratings yet
- Haloalkanes and Haloarenes Class 12 Notes Chemistry Chapter 10 - Learn CBSEDocument1 pageHaloalkanes and Haloarenes Class 12 Notes Chemistry Chapter 10 - Learn CBSEUnknownNo ratings yet
- 046 Transition Metals 2 No Answer Chen Fact SheetDocument2 pages046 Transition Metals 2 No Answer Chen Fact SheetlicciNo ratings yet
- Tarea 4Document1 pageTarea 4Lorena ContrerasNo ratings yet
- CHEM 3L Group 4 Cation 1Document6 pagesCHEM 3L Group 4 Cation 1Beatrice AlejeNo ratings yet
- 49crsbfile BSC (H) CHMCBCS2020-21 PDFDocument80 pages49crsbfile BSC (H) CHMCBCS2020-21 PDFJosephine TorresNo ratings yet
- Soil and Water Chemistry - An Integrative Approach - Essington, Michael E - CRC Press (2015)Document658 pagesSoil and Water Chemistry - An Integrative Approach - Essington, Michael E - CRC Press (2015)Abel Eloy Vargas MamaniNo ratings yet
- Delhi Public School Bangalore North (2022 - 23)Document4 pagesDelhi Public School Bangalore North (2022 - 23)itz gamerNo ratings yet
- 1.3 Answers To ExercisesDocument2 pages1.3 Answers To ExercisesAli MakiNo ratings yet
- Modern Periodic TableDocument4 pagesModern Periodic TableNabil Abdullah0% (1)
- Gen. Chemistry 1: Quarter 1 - Module 2Document26 pagesGen. Chemistry 1: Quarter 1 - Module 2AniahsNelet80% (5)
- Dental Casting Alloys FinalDocument175 pagesDental Casting Alloys FinalNiaz Ahammed0% (1)
- Salcare Super 7 at 1Document1 pageSalcare Super 7 at 1xeon585100% (1)