Professional Documents
Culture Documents
Describe and Explain How Lithium Forms An Ionic Compound
Uploaded by
Anonymous0 ratings0% found this document useful (0 votes)
9 views1 pageLithium forms an ionic compound by losing one electron to become positively charged Li+, while fluorine gains this electron to become negatively charged F-. The opposite charges of Li+ and F- ions attract each other electrostatically, forming an ionic bond and the ionic compound lithium fluoride.
Original Description:
Original Title
Ionic bonding explanation
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentLithium forms an ionic compound by losing one electron to become positively charged Li+, while fluorine gains this electron to become negatively charged F-. The opposite charges of Li+ and F- ions attract each other electrostatically, forming an ionic bond and the ionic compound lithium fluoride.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
9 views1 pageDescribe and Explain How Lithium Forms An Ionic Compound
Uploaded by
AnonymousLithium forms an ionic compound by losing one electron to become positively charged Li+, while fluorine gains this electron to become negatively charged F-. The opposite charges of Li+ and F- ions attract each other electrostatically, forming an ionic bond and the ionic compound lithium fluoride.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
Describe and explain how lithium forms an ionic compound
Your answer:
Lithium loses one electron to form an ion. The electron is not lost but transferred to fluorine to for an
ion. Lithium is a positively charged ion and fluorine is a negatively charged ion. These are oppositely
charged ions and are electrostatically attracted to one another. This is an ionic bond. Lithium fluoride is
an ionic compound because it is held intact by ionic bonds.
You might also like
- Ch-3 ElectrolysisDocument6 pagesCh-3 ElectrolysisjpsridharNo ratings yet
- The Process of Electrolysis: Worksheet 4.12: Chapter 4: Chemical ChangesDocument1 pageThe Process of Electrolysis: Worksheet 4.12: Chapter 4: Chemical ChangesJump SkillNo ratings yet
- Gangsta Granny: By: Arianna ToledoDocument5 pagesGangsta Granny: By: Arianna ToledoAnonymousNo ratings yet
- Chemistry Book SummaryDocument2 pagesChemistry Book SummaryTabeaNo ratings yet
- Chemical Bonding: 1.1. ElectronegativityDocument3 pagesChemical Bonding: 1.1. ElectronegativitysuccessNo ratings yet
- Electrolysis: 1 of 6 © Boardworks LTD 2014Document6 pagesElectrolysis: 1 of 6 © Boardworks LTD 2014Angela GarzaNo ratings yet
- Module 1 Electronic Structure and Covalent Bonding Lecture 1 Structure and Bonding IDocument19 pagesModule 1 Electronic Structure and Covalent Bonding Lecture 1 Structure and Bonding IJithin Raphi KollannurNo ratings yet
- Structure & Bonding - IONIC BONDINGDocument23 pagesStructure & Bonding - IONIC BONDINGTrishana GreenNo ratings yet
- Chemical BondingDocument60 pagesChemical BondingCacey Daiwey CalixtoNo ratings yet
- Ion and CompoundsDocument2 pagesIon and Compoundsvijay kumar honnaliNo ratings yet
- Ions and Ionic Compounds-NotesDocument3 pagesIons and Ionic Compounds-NotesKiran PandeyNo ratings yet
- Ionic Bond PresentationDocument4 pagesIonic Bond Presentationainul sofeaNo ratings yet
- Ionic and Covalent Bonds Notes: Essential Question: What Are Ions and How Are They Formed?Document31 pagesIonic and Covalent Bonds Notes: Essential Question: What Are Ions and How Are They Formed?Gerlie VelascoNo ratings yet
- Design Lab: Investigating Properties of Ionic and Covalent Compounds Using Commonly Used CompoundsDocument2 pagesDesign Lab: Investigating Properties of Ionic and Covalent Compounds Using Commonly Used Compoundsdhairya gandhiNo ratings yet
- Ionic Bond (Information)Document1 pageIonic Bond (Information)Justin Adriel Sison ApuntarNo ratings yet
- Ionic Bonding and StructureDocument20 pagesIonic Bonding and StructureKaren OrlanskiNo ratings yet
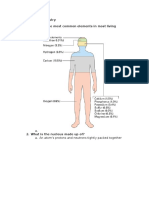
- Chapter 4 Chemistry 1. What Are The Most Common Elements in Most Living Things?Document3 pagesChapter 4 Chemistry 1. What Are The Most Common Elements in Most Living Things?Uni CornNo ratings yet
- L I L I: Chemical BondingDocument3 pagesL I L I: Chemical BondingMark IanNo ratings yet
- Ions & Ionic Bonds (Multiple Choice) QPDocument7 pagesIons & Ionic Bonds (Multiple Choice) QPBăng Băng LêNo ratings yet
- Bonding and Structure Revision CardDocument1 pageBonding and Structure Revision Cardagentdog175No ratings yet
- Ions and Ionic BondDocument21 pagesIons and Ionic Bondchickuwa pawawawaNo ratings yet
- Two Classes of Compounds: Covalent Bonding Between Hydrogen Atomssince Each Hydrogen Atom Has OneDocument2 pagesTwo Classes of Compounds: Covalent Bonding Between Hydrogen Atomssince Each Hydrogen Atom Has OnetaeNo ratings yet
- ElectrochemistryDocument8 pagesElectrochemistryTeandraNo ratings yet
- 2.3 - Notes - 12042023 - 112138Document5 pages2.3 - Notes - 12042023 - 112138Prince DanielNo ratings yet
- Class 10 Chemistry Chapter 1 Revision NotesDocument3 pagesClass 10 Chemistry Chapter 1 Revision NotesMd TaaseenNo ratings yet
- Science 9 Wlas QTR 2 Week 2 ValidatedDocument10 pagesScience 9 Wlas QTR 2 Week 2 ValidatedMYLENE B. ZABALLERONo ratings yet
- Chemistry Syllabus Notes 5072 Periodic Table of ElementsDocument3 pagesChemistry Syllabus Notes 5072 Periodic Table of ElementsJereme CheongNo ratings yet
- Bonding and FormulaDocument8 pagesBonding and FormulaJosephat MugumbaNo ratings yet
- Grade-11 Chemistry Definitions CollectionDocument65 pagesGrade-11 Chemistry Definitions CollectionMoun Lynn Sythu100% (3)
- Bonding and StructureDocument20 pagesBonding and StructureYusma KhanNo ratings yet
- Ib CHEM Topic 4 Chemical BondingDocument45 pagesIb CHEM Topic 4 Chemical Bondingyasser khairyNo ratings yet
- Chemical Bonding and PropertiesDocument21 pagesChemical Bonding and PropertiesRoshan Jawad ZafirNo ratings yet
- Electrolysis 1 TEDocument5 pagesElectrolysis 1 TETom TommmaNo ratings yet
- GCFGCGCFGFDGDocument15 pagesGCFGCGCFGFDGZabrinaRuizNo ratings yet
- 7.1 7.2Document2 pages7.1 7.2noora.rami48No ratings yet
- Lithium Battery and It ComponentsDocument4 pagesLithium Battery and It Components126009071No ratings yet
- 3-Ionic Bonding PDFDocument2 pages3-Ionic Bonding PDFTahmeed AhmedNo ratings yet
- PolarityDocument20 pagesPolarityYsabela MataNo ratings yet
- Atoms CombiningDocument12 pagesAtoms Combiningshehryar khanNo ratings yet
- Chemical BondingDocument5 pagesChemical BondingSANDEEP SINGHNo ratings yet
- Ionic and Covalent BondingDocument2 pagesIonic and Covalent BondingInamjazbiHaqNo ratings yet
- Ionic BondDocument12 pagesIonic Bondsacheetha giriNo ratings yet
- Ions and Ionic BondsDocument6 pagesIons and Ionic BondsSadiya ShaikhNo ratings yet
- Ionic BondsDocument8 pagesIonic BondsJustin Adriel Sison ApuntarNo ratings yet
- Biochemistry 2nd SemesterDocument46 pagesBiochemistry 2nd SemesterEmelly Galvez PadillaNo ratings yet
- Ionic and Covalent Bonding: By: Andrew M. Famero, BSN, RN, Maed ScienceDocument16 pagesIonic and Covalent Bonding: By: Andrew M. Famero, BSN, RN, Maed ScienceEllah Jean BalidioNo ratings yet
- Ionic Bonding 3A1 NotesDocument9 pagesIonic Bonding 3A1 NotesmunasheNo ratings yet
- Chemical Bonding Lesson OneDocument9 pagesChemical Bonding Lesson OneDNA OasisNo ratings yet
- Electron AffinityDocument15 pagesElectron AffinityOyananasha Ron100% (1)
- Introduction To BondingDocument9 pagesIntroduction To BondingTheonie DavisNo ratings yet
- Chapter 3 Chemical BondingDocument6 pagesChapter 3 Chemical BondingQutub KhanNo ratings yet
- Chem IGCSE 1 - Module 4Document4 pagesChem IGCSE 1 - Module 4carrisanicole2No ratings yet
- Lesson Sa ScienceDocument31 pagesLesson Sa Sciencejhon reyann espraNo ratings yet
- Chemistry-Ch 3 - Chemical BondingDocument8 pagesChemistry-Ch 3 - Chemical BondingHassan RiazNo ratings yet
- Cambridge IGCSE Chemistry Topic 3: Atoms, Elements and CompoundsDocument3 pagesCambridge IGCSE Chemistry Topic 3: Atoms, Elements and CompoundsStudy GuyNo ratings yet
- 3.2.2. Ions and Ionic Bonds PDFDocument3 pages3.2.2. Ions and Ionic Bonds PDFClinton ChikengezhaNo ratings yet
- Lewis Structures of IonsDocument2 pagesLewis Structures of IonsYukih GadorNo ratings yet
- CHEMICAL EFECTS OF ELECTRIC CURRENT ,,,,,,,, Topic - ELECTROLYTEDocument5 pagesCHEMICAL EFECTS OF ELECTRIC CURRENT ,,,,,,,, Topic - ELECTROLYTESanjaya SahooNo ratings yet
- Ionic CompoundsDocument2 pagesIonic CompoundsShenneth De CastroNo ratings yet
- Drawing Ions: S3 IGCSE Chemistry Topic 1: Ionic BondingDocument1 pageDrawing Ions: S3 IGCSE Chemistry Topic 1: Ionic BondingAnonymousNo ratings yet
- Investigating The Effect of Concentration On The Rate of ReactionDocument4 pagesInvestigating The Effect of Concentration On The Rate of ReactionAnonymousNo ratings yet
- Writing & Naming Ionic FormulaeDocument1 pageWriting & Naming Ionic FormulaeAnonymousNo ratings yet
- Naming Ionic CompundsDocument1 pageNaming Ionic CompundsAnonymousNo ratings yet
- Helen Keller A Woman To RememberDocument2 pagesHelen Keller A Woman To RememberAnonymousNo ratings yet
- Oceania Thought Police Department Sworn Confession: Winston SDocument2 pagesOceania Thought Police Department Sworn Confession: Winston SAnonymousNo ratings yet
- Helen Keller: By: Arianna ToledoDocument1 pageHelen Keller: By: Arianna ToledoAnonymousNo ratings yet
- Russia, 1905-41 - Terror + PurgesDocument11 pagesRussia, 1905-41 - Terror + PurgesAnonymousNo ratings yet