Professional Documents
Culture Documents
Investigating The Effect of Concentration On The Rate of Reaction
Uploaded by
AnonymousOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Investigating The Effect of Concentration On The Rate of Reaction
Uploaded by
AnonymousCopyright:
Available Formats
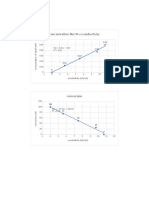
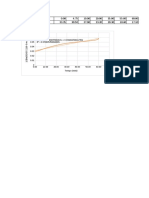
How does changing the concentration of the sodium thiosulphate affect the rate of reaction between HCl and
Na₂S₂O₃?
0.006
0.005
0.004
Rate of reaction (1/S)
0.003
0.002
0.001
0
0.04 0.05 0.06 0.07 0.08 0.09 0.10 0.11
Concentration of sodium thiosulphate (M) ± 10%
How does changing the concentration of the sodium thiosulphate affect the time taken for the X to disappear?
600.00
500.00
400.00
Time taken for X to disappear (s)
300.00
200.00
100.00
0.00
0.04 0.05 0.06 0.07 0.08 0.09 0.10 0.11
Concentration of sodium thiosulphate (M) ± 10%
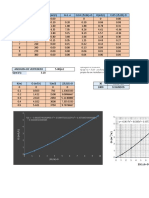
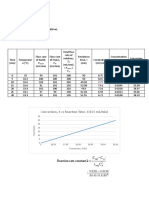
Investigating the effect of concentration on the rate of reaction
Volume (ml) ± 0.01 Time for X to disappear (min) ± 0.01 Rate
2HCl Na₂S₂O₃ H₂O T1 T2 T3 Average (1/S)
10 10 0 3.02 3.22 3.17 3.14 0.318810
10 9 1 3.37 4.24 3.58 3.73 0.268097
10 8 2 4.47 5.31 5.05 4.94 0.202293
10 7 3 5.49 5.55 6.03 5.69 0.175747
10 6 4 9.02 7.38 7.53 7.98 0.125366
10 5 5 9.22 8.50 8.59 8.77 0.114025
Investigating the effect of concentration on the rate of reaction
Volume (ml) ± 0.01 Time for X to disappear (s) ± 0.01 Rate
2HCl Na₂S₂O₃ H₂O T1 T2 T3 Average (1/S)
10 10 0 181.20 193.20 190.20 188.20 0.0053
10 9 1 202.20 254.40 214.80 223.80 0.0045
10 8 2 268.20 318.60 303.00 296.60 0.0034
10 7 3 329.40 333.00 361.80 341.40 0.0029
10 6 4 541.20 442.80 451.80 478.60 0.0021
10 5 5 553.20 510.00 515.40 526.20 0.0019
Concentration of Rate of Reaction
Na₂S₂O₃ (M) ± 10% (1/S)
0.10 0.0053
0.09 0.0045
0.08 0.0034
0.07 0.0029
0.06 0.0021
0.05 0.0019
Concentration of Average time for X to
Na₂S₂O₃ (M) ± 10% disappear (s) ± 0.01
0.10 188.20
0.09 223.80
0.08 296.60
0.07 341.40
0.06 478.60
0.05 526.20
You might also like
- Test Bank For Organic Chemistry 11th Edition Francis Carey Robert Giuliano Janice SmithDocument32 pagesTest Bank For Organic Chemistry 11th Edition Francis Carey Robert Giuliano Janice Smithxavianhatmgzmz9No ratings yet
- Formulation-Book KabooDocument65 pagesFormulation-Book KabooMiezha Lecter Vengerberg100% (1)
- Lab Report Batch Reactor GGDocument25 pagesLab Report Batch Reactor GGFrost Orchid100% (1)
- Gangsta Granny: By: Arianna ToledoDocument5 pagesGangsta Granny: By: Arianna ToledoAnonymousNo ratings yet
- Result Analysis: Naoh EtDocument7 pagesResult Analysis: Naoh EtSuria Seri SulyanaNo ratings yet
- KimiaaaaaaDocument11 pagesKimiaaaaaaaimi BatrisyiaNo ratings yet
- Of 0.1M Na S O Solution (ML) of Distilled Water (ML) Concent Ration of Na S O (M) of 0.1M HCL Solution (ML) Time, T (S) 1/T (S)Document4 pagesOf 0.1M Na S O Solution (ML) of Distilled Water (ML) Concent Ration of Na S O (M) of 0.1M HCL Solution (ML) Time, T (S) 1/T (S)aimi BatrisyiaNo ratings yet
- Batch ReactorDocument16 pagesBatch ReactorZharifah Bari'ah Basa'ahNo ratings yet
- Hid Rome TriaDocument3 pagesHid Rome TriaDavid BolivarNo ratings yet
- Calculos Lab 1Document5 pagesCalculos Lab 1japerez67 japerez67No ratings yet
- No Naoh (ML) K (MS/CM) Rata - Rata Data 1 Data 2Document8 pagesNo Naoh (ML) K (MS/CM) Rata - Rata Data 1 Data 2Teknik Kimia B 2016No ratings yet
- RTD Cobined PFR CSTRDocument5 pagesRTD Cobined PFR CSTRhanamant jamadarNo ratings yet
- Potato Osmolarity IA - ) ) 2Document11 pagesPotato Osmolarity IA - ) ) 2Kanika KumarNo ratings yet
- Concentration Naoh Vs Conductivity: 2.0 ResultsDocument6 pagesConcentration Naoh Vs Conductivity: 2.0 ResultsnisasoberiNo ratings yet
- Result Exp 4Document3 pagesResult Exp 4M Asrar SidonNo ratings yet
- Sample Number Sample Time Elapsed Time Sodium Hydroxide Concentaion (Mol/dm ) Ethyl Acetate Concentraio N (Mol/dm )Document5 pagesSample Number Sample Time Elapsed Time Sodium Hydroxide Concentaion (Mol/dm ) Ethyl Acetate Concentraio N (Mol/dm )Kelly Sheine SisonNo ratings yet
- Sample Number Sample Time Elapsed Time Sodium Hydroxide Concentaion (Mol/dm ) Ethyl Acetate Concentraio N (Mol/dm )Document5 pagesSample Number Sample Time Elapsed Time Sodium Hydroxide Concentaion (Mol/dm ) Ethyl Acetate Concentraio N (Mol/dm )Kelly Sheine SisonNo ratings yet
- Sodium ThiosulfateDocument8 pagesSodium ThiosulfateJakeNo ratings yet
- Temperature (Degrees Celcius)Document3 pagesTemperature (Degrees Celcius)Chloe UyNo ratings yet
- CPP Lab 1 MuahDocument16 pagesCPP Lab 1 Muahelynnmasrof100% (1)
- TrabajoDocument4 pagesTrabajoSofía RivasNo ratings yet
- VariasiDocument2 pagesVariasidickyNo ratings yet
- L52 Online Assignment Question 1Document3 pagesL52 Online Assignment Question 1Raj PatelNo ratings yet
- Preci. (MM) Int (MM/HR)Document5 pagesPreci. (MM) Int (MM/HR)Alejandro P. MartinezNo ratings yet
- Determination of Degree of Micelle IonisationDocument5 pagesDetermination of Degree of Micelle IonisationthikamenituyeniNo ratings yet
- Rate Reaction Lloaana 2020Document8 pagesRate Reaction Lloaana 2020Lloaana 12No ratings yet
- Areas Aportantes MCTDocument16 pagesAreas Aportantes MCTRicardo Díaz G.No ratings yet
- PH Vs Tiempo: PH Tiempo (Min) Gasto de Agno3 (ML) Nacn Inicial (PPM)Document3 pagesPH Vs Tiempo: PH Tiempo (Min) Gasto de Agno3 (ML) Nacn Inicial (PPM)leslie casaicoNo ratings yet
- Grafik UtsDocument8 pagesGrafik UtsRodi AnaNo ratings yet
- Enzyme ActivityDocument3 pagesEnzyme ActivitySyarmine Aqila IsaNo ratings yet
- Standard CurveDocument2 pagesStandard CurveNor SyuhailaNo ratings yet
- Core Practical 1 ChemistryDocument4 pagesCore Practical 1 ChemistryAadharsh NandhakumarNo ratings yet
- NLSJNDKLSNKLSJDocument2 pagesNLSJNDKLSNKLSJPutri Pah Kumala DewiNo ratings yet
- Anthony M. Wachinski - Environmental Ion Exchange - Principles and Design-Taylor & Francis, Chapman and Hall - CRC (2016) (1) (138-157)Document20 pagesAnthony M. Wachinski - Environmental Ion Exchange - Principles and Design-Taylor & Francis, Chapman and Hall - CRC (2016) (1) (138-157)HARDY EDDISONNo ratings yet
- Chemical Treatment CalculationDocument1 pageChemical Treatment CalculationNghiaNo ratings yet
- Water Treatment Volume Calculation Size (Inch) Legnth (MT) Area (M 2) Volume (M 3)Document1 pageWater Treatment Volume Calculation Size (Inch) Legnth (MT) Area (M 2) Volume (M 3)Robert V. AbrasaldoNo ratings yet
- Water TreatementDocument1 pageWater TreatementSayeed HvacNo ratings yet
- Water Treatment Volume Calculation Size (Inch) Legnth (MT) Area (M 2) Volume (M 3)Document1 pageWater Treatment Volume Calculation Size (Inch) Legnth (MT) Area (M 2) Volume (M 3)Raju KsnNo ratings yet
- Water Treatment Volume Calculation Size (Inch) Legnth (MT) Area (M 2) Volume (M 3)Document1 pageWater Treatment Volume Calculation Size (Inch) Legnth (MT) Area (M 2) Volume (M 3)Raju KsnNo ratings yet
- Water Treatment Volume Calculation Size (Inch) Legnth (MT) Area (M 2) Volume (M 3)Document1 pageWater Treatment Volume Calculation Size (Inch) Legnth (MT) Area (M 2) Volume (M 3)Raju KsnNo ratings yet
- Water Treatment Volume Calculation Size (Inch) Legnth (MT) Area (M 2) Volume (M 3)Document1 pageWater Treatment Volume Calculation Size (Inch) Legnth (MT) Area (M 2) Volume (M 3)Raju KsnNo ratings yet
- Water TreatementDocument1 pageWater Treatementmehmet akildizNo ratings yet
- Water TreatementDocument1 pageWater Treatementmehmet akildizNo ratings yet
- GraficaDocument1 pageGraficaAnaPatriciaCardenasQuezadaNo ratings yet
- Assignment 1 Momentum Transfer l01 Wan Khairul Azmi & Suraya Binti JohariDocument9 pagesAssignment 1 Momentum Transfer l01 Wan Khairul Azmi & Suraya Binti JohariSuraya JohariNo ratings yet
- ADARNe CerveraAndreaDocument8 pagesADARNe CerveraAndreaAndrea CerveraNo ratings yet
- Lectura # D0 (MM) DC (MM) H0 (MM) HC (MM) V (LTS) V (M )Document4 pagesLectura # D0 (MM) DC (MM) H0 (MM) HC (MM) V (LTS) V (M )Hanssel Jerome Mayorga MoragaNo ratings yet
- Concentration Naoh Vs ConductivityDocument10 pagesConcentration Naoh Vs ConductivitynisasoberiNo ratings yet
- GraphDocument10 pagesGraphnisasoberiNo ratings yet
- PERHITUNGAN - EXCELDocument12 pagesPERHITUNGAN - EXCELNesty YomanNo ratings yet
- Results: Conversion, X Vs Reaction Time, T (100 Ml/min)Document9 pagesResults: Conversion, X Vs Reaction Time, T (100 Ml/min)Rashdan CskNo ratings yet
- LN (LN (1/ (1-f (T) ) : F (X) 2.6454117725x - 11.1626312721 R 0.8889049032Document10 pagesLN (LN (1/ (1-f (T) ) : F (X) 2.6454117725x - 11.1626312721 R 0.8889049032Jhonatan Junior Villasante RoqueNo ratings yet
- F4401201032 Putri Nadia Teja P2 Penelusuran Banjir (1) Praktikum 14Document3 pagesF4401201032 Putri Nadia Teja P2 Penelusuran Banjir (1) Praktikum 14Putri Nadia Teja F4-32No ratings yet
- Well Function W (U) - UDocument8 pagesWell Function W (U) - URendy Khoirul IlhamNo ratings yet
- 6.1 Cuy-Mix-20120Document4 pages6.1 Cuy-Mix-20120Aurora Grundy pintoNo ratings yet
- Perhitungan Iodin NumberDocument268 pagesPerhitungan Iodin NumberrusydianaabdNo ratings yet
- Gráficos Da Lei Da Velocidade IntegradaDocument5 pagesGráficos Da Lei Da Velocidade IntegradaYara Fernandes FlemingNo ratings yet
- Concentration vs. AbsorbanceDocument4 pagesConcentration vs. AbsorbanceEllah GutierrezNo ratings yet
- Soil Testing CalculationDocument5 pagesSoil Testing CalculationAmit Kumar PaulNo ratings yet
- Program Pondasi Tiang Berdasarkan Data NSPTDocument4 pagesProgram Pondasi Tiang Berdasarkan Data NSPTSyarifudinBahriKromowijoyoNo ratings yet
- Result TPP EXPERIMENT 2Document4 pagesResult TPP EXPERIMENT 2syahmisivNo ratings yet
- Data Praktikum Isoterm AdsorpsiDocument7 pagesData Praktikum Isoterm Adsorpsirudi salamNo ratings yet
- Math Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesFrom EverandMath Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesRating: 5 out of 5 stars5/5 (3)
- Describe and Explain How Lithium Forms An Ionic CompoundDocument1 pageDescribe and Explain How Lithium Forms An Ionic CompoundAnonymousNo ratings yet
- Drawing Ions: S3 IGCSE Chemistry Topic 1: Ionic BondingDocument1 pageDrawing Ions: S3 IGCSE Chemistry Topic 1: Ionic BondingAnonymousNo ratings yet
- Naming Ionic CompundsDocument1 pageNaming Ionic CompundsAnonymousNo ratings yet
- Writing & Naming Ionic FormulaeDocument1 pageWriting & Naming Ionic FormulaeAnonymousNo ratings yet
- Helen Keller A Woman To RememberDocument2 pagesHelen Keller A Woman To RememberAnonymousNo ratings yet
- Oceania Thought Police Department Sworn Confession: Winston SDocument2 pagesOceania Thought Police Department Sworn Confession: Winston SAnonymousNo ratings yet
- Helen Keller: By: Arianna ToledoDocument1 pageHelen Keller: By: Arianna ToledoAnonymousNo ratings yet
- Russia, 1905-41 - Terror + PurgesDocument11 pagesRussia, 1905-41 - Terror + PurgesAnonymousNo ratings yet
- Cambridge Assessment International Education: Chemistry 9701/42 May/June 2019Document13 pagesCambridge Assessment International Education: Chemistry 9701/42 May/June 2019Ali110No ratings yet
- Acetylene How Products Are MadeDocument3 pagesAcetylene How Products Are MadeJoy MukherjeNo ratings yet
- Experiment 9 - Synthesis & Analysis of Biodiesel From Vegetable Oil Via TransesterificationDocument3 pagesExperiment 9 - Synthesis & Analysis of Biodiesel From Vegetable Oil Via TransesterificationLindelwa MthembuNo ratings yet
- CHEM 212 Laboratory Experiment 6Document6 pagesCHEM 212 Laboratory Experiment 6MinaBaNo ratings yet
- Lubricants in Refrigerant Systems: Related Commercial ResourcesDocument29 pagesLubricants in Refrigerant Systems: Related Commercial ResourcesAndrés Felipe NaranjoNo ratings yet
- 0620 s21 QP 33 PDFDocument16 pages0620 s21 QP 33 PDFTshegofatso SaliNo ratings yet
- Parker Chomerics PRO SHIELD Recommended Surface PreparationDocument3 pagesParker Chomerics PRO SHIELD Recommended Surface PreparationRanjeetNo ratings yet
- Chemistry 2022Document4 pagesChemistry 2022New Prestige WelfareNo ratings yet
- BeautyForward II - BALANCE - Hair Fiber Firewall Curvy HairDocument1 pageBeautyForward II - BALANCE - Hair Fiber Firewall Curvy HairAngelica Hurtado CollanteNo ratings yet
- Isolation of Ecgonidine Methyl Ester From Coca SeedDocument3 pagesIsolation of Ecgonidine Methyl Ester From Coca SeedJames McNeeNo ratings yet
- Jurnal Injeksi PhenoDocument10 pagesJurnal Injeksi Phenoastriani oktaviaNo ratings yet
- Vanish: WWW - Chemistry.co - Nz/stain - Frame - HTM)Document15 pagesVanish: WWW - Chemistry.co - Nz/stain - Frame - HTM)Mohammad MariasaNo ratings yet
- Soap and detergent-KMMDocument5 pagesSoap and detergent-KMMMd KhanNo ratings yet
- Environmental Monitoring PlanDocument7 pagesEnvironmental Monitoring Planyemi adelakunNo ratings yet
- Daftar Pustaka - 1Document3 pagesDaftar Pustaka - 1FaniNo ratings yet
- Pro TR-144S 5.4.21Document8 pagesPro TR-144S 5.4.21Manufaktur Sinar JoyoboyoNo ratings yet
- Carrier Dyeing Method of Disperse DyeDocument2 pagesCarrier Dyeing Method of Disperse DyerashidtexNo ratings yet
- Quantitative Analysis of Coconut WaterDocument13 pagesQuantitative Analysis of Coconut WaterAditya Mishra100% (1)
- CSEC Chemistry - Acids, Bases and SaltsDocument4 pagesCSEC Chemistry - Acids, Bases and SaltsCornflakes ToastedNo ratings yet
- THC24 Phen HalDocument32 pagesTHC24 Phen Halalifiya nur rosidahNo ratings yet
- Field Expedient Methods For Explosives Preparation - 5ac3733a1723dd9445078f1bDocument9 pagesField Expedient Methods For Explosives Preparation - 5ac3733a1723dd9445078f1bTeleson MarquesNo ratings yet
- Lonsum Budplan 2014 (Estate)Document4,771 pagesLonsum Budplan 2014 (Estate)Achmad Jerry Rizky100% (1)
- Lab ReportDocument8 pagesLab Reportapi-392375614No ratings yet
- Salt Effect in Distillation - A Literature ReviewDocument14 pagesSalt Effect in Distillation - A Literature Reviewcombo162No ratings yet
- Lactic Acid Recent Advances in ProductsDocument11 pagesLactic Acid Recent Advances in ProductsMathilda PasaribuNo ratings yet
- S - Block Elements PDFDocument14 pagesS - Block Elements PDFPankaj MauryaNo ratings yet
- Metallurgical ManganeseDocument24 pagesMetallurgical ManganeseBoniface SinghNo ratings yet
- Chemistry, Tenth Edition (PDFDrive) - 100 - 100Document10 pagesChemistry, Tenth Edition (PDFDrive) - 100 - 100Ezat JrNo ratings yet