Professional Documents
Culture Documents
L52 Online Assignment Question 1
Uploaded by
Raj Patel0 ratings0% found this document useful (0 votes)
6 views3 pagesF
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentF
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views3 pagesL52 Online Assignment Question 1
Uploaded by
Raj PatelF
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 3
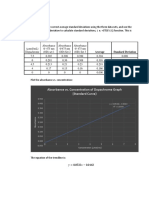
0.050 L HNO₃ Vol NaOH (mL) (0.
1781M) Moles NaOH Moles HNO₃ Molarity HNO₃
Trial 1 22.56 0.004018 0.004018 0.08036
Trial 2 23.01 0.004098 0.004098 0.08196
Trial 3 22.82 0.004064 0.004064 0.08128
Trial 4 22.73 0.004048 0.004048 0.08096
Average M 0.08114
Std. Dev. M 0.0006677
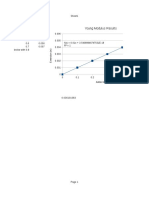
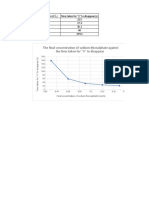
Concentration (parts per million, ppm) Absorbance
20 0.836
15 0.556
10 0.402
5 0.206
2 0.091
0 0.000
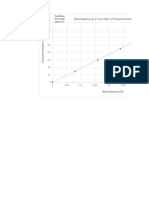
Absorbance vs. Concentration
0.9
0.8
0.7 y = 0.0401x + 0.0008
R² = 0.9931
Absorbance
0.6
0.5
0.4
0.3
0.2
0.1
0
0 5 10 15 20 25
Concentration (ppm)
Due to the fact that 0.9931 is the R^2 value and that it is close to 1, there is a high or
strong correlation between the absorbance (dependent variable) and concentration
(independent variable). This also means that 99.31% of the variance of the
absorbance is explained by the variance of the concentration.
You might also like
- Lab Report Batch Reactor GGDocument25 pagesLab Report Batch Reactor GGFrost Orchid100% (1)
- Determination of Nitrate in Wastewater Using Sodium SalicylateDocument13 pagesDetermination of Nitrate in Wastewater Using Sodium SalicylateJuan ManuelNo ratings yet
- Concentration vs. AbsorbanceDocument4 pagesConcentration vs. AbsorbanceEllah GutierrezNo ratings yet
- Experiment 2Document18 pagesExperiment 2api-3868874100% (1)
- Residence Time Distribution Analysis of A Continuous Stirred Tank ReactorDocument10 pagesResidence Time Distribution Analysis of A Continuous Stirred Tank ReactorNurul IzzahNo ratings yet
- Relationship Between Conductivity and NaOH ConcentrationDocument8 pagesRelationship Between Conductivity and NaOH ConcentrationSalihah AbdullahNo ratings yet
- Standard GraphDocument7 pagesStandard GraphVAISHNAVI JADHAVNo ratings yet
- Penentuan Uji Kelarutan KTI ParacetamolDocument6 pagesPenentuan Uji Kelarutan KTI ParacetamolMinul1412No ratings yet
- Lab AnalysisDocument4 pagesLab AnalysisErnestasBlaževičNo ratings yet
- Abs Vs Concentration of MN: 1.2 F (X) 0.0099552x + 0.0265 R 0.9913511755Document2 pagesAbs Vs Concentration of MN: 1.2 F (X) 0.0099552x + 0.0265 R 0.9913511755ashNo ratings yet
- Practica de Laboratorio 4Document2 pagesPractica de Laboratorio 4Leidy Renteria EstradaNo ratings yet
- Absorbance at λmax 260nm for PBS: Calibration curvesDocument3 pagesAbsorbance at λmax 260nm for PBS: Calibration curvesNaveedNo ratings yet
- Measuring glucose concentration using a spectrophotometerDocument2 pagesMeasuring glucose concentration using a spectrophotometerSebastian SmytheNo ratings yet
- Accuracy Percision LabDocument2 pagesAccuracy Percision LabAlexaNo ratings yet
- trk6Document5 pagestrk6retnoNo ratings yet
- Kurva Kalbrasi Waktu TampakDocument1 pageKurva Kalbrasi Waktu Tampakirzaaprilian12No ratings yet
- Kurva Larutan Standar GlukosaDocument2 pagesKurva Larutan Standar GlukosaTiara RiaNo ratings yet
- Relationship Between Drug Concentration and TimeDocument4 pagesRelationship Between Drug Concentration and TimeAnnisa Fauzia UlhaqNo ratings yet
- Concentration vs Absorbance of Cu+2 Graph and CalculationsDocument2 pagesConcentration vs Absorbance of Cu+2 Graph and Calculationsmiami14No ratings yet
- Data Analisis TranslasiDocument17 pagesData Analisis TranslasiAkhmd RdlNo ratings yet
- Waste Water Testing Data SheetDocument3 pagesWaste Water Testing Data SheetAffan MahdyNo ratings yet
- Psychrometric Analyzer Version 6.8 by Mcquay InternationalDocument1 pagePsychrometric Analyzer Version 6.8 by Mcquay InternationalLi LiuNo ratings yet
- Aspirin Callibration CurveDocument2 pagesAspirin Callibration Curveirfanh15951No ratings yet
- Tugas 1 - Eka Kurniati Kombong Datu - 05201026Document9 pagesTugas 1 - Eka Kurniati Kombong Datu - 05201026시우민SeohyunNo ratings yet
- Lab 4Document3 pagesLab 4Olivia PowerNo ratings yet
- Exepermint 1Document4 pagesExepermint 1Jhone SaaimonNo ratings yet
- Absorbance BB at 618 NM: Absorbance Molarity (Mol L)Document16 pagesAbsorbance BB at 618 NM: Absorbance Molarity (Mol L)Stegi IlanthiraianNo ratings yet
- My WorkDocument3 pagesMy WorkajecultureNo ratings yet
- Spectrophotometric Study of Complexes by Job's Method: Report of The Minor UGC Project EntitledDocument19 pagesSpectrophotometric Study of Complexes by Job's Method: Report of The Minor UGC Project EntitledM Irfan Khan100% (1)
- Experiment 5 - Data TreatmentDocument6 pagesExperiment 5 - Data TreatmentShawn Ann SilanNo ratings yet
- Curva CalibracionDocument2 pagesCurva CalibracionSofiaNo ratings yet
- Kurva Kalibrasi Parasetamol λ maks 243 nmDocument2 pagesKurva Kalibrasi Parasetamol λ maks 243 nmGelisaNo ratings yet
- Bondocch4810 ActivitycoefficientDocument4 pagesBondocch4810 ActivitycoefficientTope BondocNo ratings yet
- ChimieDocument2 pagesChimieRaymond el hachemNo ratings yet
- Kurva Hubungan Konsentrasi Dengan Absorbans Sesudah FotokatalisisDocument2 pagesKurva Hubungan Konsentrasi Dengan Absorbans Sesudah FotokatalisisMuhammad AksanNo ratings yet
- Glucose Concentr Ation (MG/L) Absorba Nce (A) : Glucose Concentration Vs AbsorbanceDocument6 pagesGlucose Concentr Ation (MG/L) Absorba Nce (A) : Glucose Concentration Vs Absorbancesyahirah shamsudinNo ratings yet
- Kurva Standar Metode Biuret Dengan Panjang Gelombang 520 NM: Konsentrasi Albumin (MG/ ML)Document1 pageKurva Standar Metode Biuret Dengan Panjang Gelombang 520 NM: Konsentrasi Albumin (MG/ ML)Teti HungkulNo ratings yet
- Spreadsheet 5Document3 pagesSpreadsheet 5DanKimberleyNo ratings yet
- Kurva: Konsentrasi Absorbansi 0 0 2 0.053 3.8 0.104 5.8 0.16 8 0.22 9.6 0.26 11.2 0.31Document2 pagesKurva: Konsentrasi Absorbansi 0 0 2 0.053 3.8 0.104 5.8 0.16 8 0.22 9.6 0.26 11.2 0.31HijrahNo ratings yet
- DataDocument6 pagesDataαγαπημένη του ΧριστούNo ratings yet
- Plots For Exp1Document4 pagesPlots For Exp1Mohammed AlmoriseyNo ratings yet
- Figures and TablesDocument3 pagesFigures and TablesAngeline RabuyoNo ratings yet
- Analisa Air LautDocument8 pagesAnalisa Air LautUfafa AnggariniNo ratings yet
- Zero Order Reaction First Order ReactionDocument1 pageZero Order Reaction First Order ReactionSanjeev NehruNo ratings yet
- Concentracion (MG/L) Absorbancia 0.20 0.014 0.50 0.032 1.00 0.065 1.50 0.108 2.00 0.135Document2 pagesConcentracion (MG/L) Absorbancia 0.20 0.014 0.50 0.032 1.00 0.065 1.50 0.108 2.00 0.135JUANMI CHIKNo ratings yet
- Enzyme Kinetic ReportDocument7 pagesEnzyme Kinetic ReportHalil Onur AltayNo ratings yet
- Determination of Trace Elements: ReagentsDocument4 pagesDetermination of Trace Elements: ReagentsGustavo Tovar GaitanNo ratings yet
- Rate of Reaction of Sodium ThiosulphateDocument3 pagesRate of Reaction of Sodium ThiosulphateLohNo ratings yet
- Enzyme ActivityDocument3 pagesEnzyme ActivitySyarmine Aqila IsaNo ratings yet
- Ammonium, Nitrit & Nitrat Levels in Water SamplesDocument9 pagesAmmonium, Nitrit & Nitrat Levels in Water SamplesRafaelNo ratings yet
- Practice Graph CHM 141 TestDocument2 pagesPractice Graph CHM 141 Testeyewannadie8No ratings yet
- Perhitungan EsterDocument6 pagesPerhitungan EsteralfinNo ratings yet
- Investigating The Effect of Concentration On The Rate of ReactionDocument4 pagesInvestigating The Effect of Concentration On The Rate of ReactionAnonymousNo ratings yet
- Sodium ThiosulfateDocument8 pagesSodium ThiosulfateJakeNo ratings yet
- Adsorption of Acetic Acid by Activated CarbonDocument11 pagesAdsorption of Acetic Acid by Activated CarbonHayden Chappelear-RobbinsNo ratings yet
- Pengolahan DataDocument12 pagesPengolahan Dataari antoNo ratings yet
- SpecDocument8 pagesSpecJirapat ThonglekpechNo ratings yet
- Glucose Standard Curve and Factors Affecting Amylase Enzyme ActivityDocument3 pagesGlucose Standard Curve and Factors Affecting Amylase Enzyme ActivityasyharulNo ratings yet