Professional Documents
Culture Documents
5.7 Ionic Compounds Worksheet
Uploaded by
jadattle050 ratings0% found this document useful (0 votes)
3 views2 pagesThe document provides worksheets for students to practice writing names and formulas for binary ionic compounds and multivalent ionic compounds. For binary ionic compounds, students are asked to write the names for given formulas and formulas for given names. For multivalent ionic compounds, students are asked to write formulas for given names indicating oxidation states and names for given formulas. The document contains practice problems for various combinations of metals and nonmetals.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document provides worksheets for students to practice writing names and formulas for binary ionic compounds and multivalent ionic compounds. For binary ionic compounds, students are asked to write the names for given formulas and formulas for given names. For multivalent ionic compounds, students are asked to write formulas for given names indicating oxidation states and names for given formulas. The document contains practice problems for various combinations of metals and nonmetals.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
3 views2 pages5.7 Ionic Compounds Worksheet
Uploaded by
jadattle05The document provides worksheets for students to practice writing names and formulas for binary ionic compounds and multivalent ionic compounds. For binary ionic compounds, students are asked to write the names for given formulas and formulas for given names. For multivalent ionic compounds, students are asked to write formulas for given names indicating oxidation states and names for given formulas. The document contains practice problems for various combinations of metals and nonmetals.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
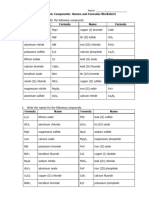
Binary Ionic Compounds:
Names and Formulas Worksheet
1. Write the names for the following compounds
(a) Li2O (g) Na3N
(b) AlCl3 (h) Al2O3
(c) MgS (i) Na2S
(d) CaO (j) Mg3P2
(e) KBr (k) CaF2
(f) BeF2 (l) K3P
2. Write the formulas for the following compounds
(a) magnesium oxide (f) calcium bromide
(b) sodium fluoride (g) beryllium oxide
(c) aluminum nitride (h) magnesium nitride
(d) potassium sulphide (i) aluminum sulphide
(e) lithium iodide (j) calcium nitride
3. Write the name and formula for the compounds formed by the following metal – non-metal combinations
Name Formula
(a) lithium and nitrogen
(b) calcium and phosphorous
(c) beryllium and bromine
(d) silver and oxygen
(e) potassium and fluorine
(f) sodium and oxygen
Multivalent Ionic Compounds:
Names and Formulas Worksheet
1. Write the formulas for the following compounds
(a) copper (I) bromide (g) copper (II) sulphide
(b) tin (II) iodide (h) iron (II) oxide
(c) iron (III) chloride (i) iron (III) oxide
(d) lead (II) oxide (j) copper (II) chloride
(e) lead (IV) fluoride (k) tin (II) chloride
(f) tin (IV) bromide (l) tin (IV) oxide
2. Write the names for the following compounds
(a) CuCl (g) PbS
(b) CuCl2 (h) FeP
(c) FeBr3 (i) FeCl3
(d) PbCl4 (j) Cu2S
(e) CuI (k) SnCl2
(f) SnO2 (l) PbBr2
You might also like
- Filler Metal SelectionDocument7 pagesFiller Metal SelectionMidhun K ChandraboseNo ratings yet
- P Block 1Document19 pagesP Block 1Sambhav Singhal100% (1)
- Types of ReactionDocument24 pagesTypes of ReactionMary joyNo ratings yet
- Penukar Ion 07Document34 pagesPenukar Ion 07Dila AdilaNo ratings yet
- Foundations of College Chemistry 14th Edition Hein Solutions Manual DownloadDocument9 pagesFoundations of College Chemistry 14th Edition Hein Solutions Manual DownloadJohn Gaudreau100% (25)
- Ionic Compound Formula Writing WorksheetDocument2 pagesIonic Compound Formula Writing WorksheetJackson LtorishaNo ratings yet
- 1.5A Ionic Compounds, Extra ExercisesDocument2 pages1.5A Ionic Compounds, Extra ExercisesDaniel StandringNo ratings yet
- 1.5B Solutions For Ionic Compounds, Extra ExercisesDocument1 page1.5B Solutions For Ionic Compounds, Extra ExercisesDaniel StandringNo ratings yet
- Nomenclature Worksheet NDocument2 pagesNomenclature Worksheet NVictor GarciaNo ratings yet
- FormulaDocument6 pagesFormulaLars RembrandtNo ratings yet
- Bonding WorksheetDocument1 pageBonding WorksheetPuffsNo ratings yet
- SaltsDocument2 pagesSaltsignacymarcinkiewicz1No ratings yet
- Ionic Compounds Names and Formulas Worksheet AnswersDocument2 pagesIonic Compounds Names and Formulas Worksheet AnswersShayan UzzamanNo ratings yet
- WS#2 Naming and Writing Inorganic CompoundsDocument6 pagesWS#2 Naming and Writing Inorganic CompoundsOw ZeeNo ratings yet
- Part - I: Only One Option Correct Type: Basic Inorganic NomenclatureDocument3 pagesPart - I: Only One Option Correct Type: Basic Inorganic NomenclaturewanderedNo ratings yet
- Kingston College Valency and Writing Formulae Home Work: Name: - Grade 10Document2 pagesKingston College Valency and Writing Formulae Home Work: Name: - Grade 1012&13 SciencesNo ratings yet
- Formula, Equation WSDocument5 pagesFormula, Equation WSAjwan YasinNo ratings yet
- Problem Set 3 NomenclatureDocument3 pagesProblem Set 3 NomenclatureKê VîňNo ratings yet
- Gallium Nitride: Ferric Sulfide Copper SelenideDocument3 pagesGallium Nitride: Ferric Sulfide Copper SelenideFernando CastilloNo ratings yet
- Lesson 1 ChemistryDocument41 pagesLesson 1 Chemistry359 Srinivasa RamanNo ratings yet
- Selina Concise Chemistry Class 9 ICSE Solutions For Chapter 1 - Language of ChemistryDocument24 pagesSelina Concise Chemistry Class 9 ICSE Solutions For Chapter 1 - Language of ChemistryfelixNo ratings yet
- Ws Naming Compounds 9-11-08Document2 pagesWs Naming Compounds 9-11-08Yahra Aquino100% (1)
- Test 1 Formula of IonsDocument6 pagesTest 1 Formula of IonsSEAW FUI MINGNo ratings yet
- Chapter 1, Naming CompoundsDocument19 pagesChapter 1, Naming CompoundsKurdishNo ratings yet
- Dokumen - Tips Chapter 3 Compounds and Molecules What Is The Correct 1020 Gmol ChapterDocument10 pagesDokumen - Tips Chapter 3 Compounds and Molecules What Is The Correct 1020 Gmol ChapterInfernape IncineroarNo ratings yet
- Nomenclature: Review ProblemsDocument1 pageNomenclature: Review ProblemsJohn Yoro ParlindunganNo ratings yet
- 1.6A Molecular Compounds, Extra ExercisesDocument1 page1.6A Molecular Compounds, Extra ExercisesDaniel StandringNo ratings yet
- Chemical Formula WorksheetDocument4 pagesChemical Formula WorksheetMaria adeelNo ratings yet
- Review #3 NomenclatureDocument1 pageReview #3 NomenclatureCassandra MachadoNo ratings yet
- Paper 04Document5 pagesPaper 04FRANCISNo ratings yet
- IB Chemistry-Revision On Naming Ionic CompoundsDocument3 pagesIB Chemistry-Revision On Naming Ionic CompoundsRania ShabanNo ratings yet
- Coordination Compounds - QuestionDocument3 pagesCoordination Compounds - Questionbest badmintonNo ratings yet
- Tutorial 2aDocument1 pageTutorial 2aAnis AzwaNo ratings yet
- Honors Chemistry WKSHT Names and Formulas V and ANSWERSDocument2 pagesHonors Chemistry WKSHT Names and Formulas V and ANSWERSkijijisellerNo ratings yet
- Coordination Compounds (Exercise+Answers)Document32 pagesCoordination Compounds (Exercise+Answers)HanukkahNo ratings yet
- Assessment Chapter 4 Group 2Document9 pagesAssessment Chapter 4 Group 2masya marchelinaNo ratings yet
- Tutorial 1aDocument1 pageTutorial 1aFatin IzzatyNo ratings yet
- CH 12 PDFDocument22 pagesCH 12 PDFkrishnaNo ratings yet
- Eleazar - Quiz#3Document2 pagesEleazar - Quiz#3ゆかりNo ratings yet
- Exercise 1a ChemistryDocument10 pagesExercise 1a Chemistryapi-533545229No ratings yet
- Post 5.9. Ionic Compounds Practice - ANSWERSDocument3 pagesPost 5.9. Ionic Compounds Practice - ANSWERSAlan MartínNo ratings yet
- Chemistry Chapter 1.exercise 1ADocument28 pagesChemistry Chapter 1.exercise 1AAsifNo ratings yet
- Review Test 2Document6 pagesReview Test 2Aditya RajputNo ratings yet
- Chemistry NotesDocument3 pagesChemistry Notesdianajose1No ratings yet
- Printout 67Document3 pagesPrintout 67dianajose1No ratings yet
- 2020 Form 3.1 Formulae HomeworkDocument1 page2020 Form 3.1 Formulae HomeworkKupakwashe KampiniNo ratings yet
- Preparation and Properties of Compounds-03 - Assignments (New)Document12 pagesPreparation and Properties of Compounds-03 - Assignments (New)Raju SinghNo ratings yet
- Nomenclature Review AssignmentDocument8 pagesNomenclature Review AssignmentTish BarnesNo ratings yet
- Chemical Formula Writing WorksheetDocument5 pagesChemical Formula Writing WorksheetÂziz ShuvoNo ratings yet
- Formulas and Names of Ionic CompoundsDocument3 pagesFormulas and Names of Ionic CompoundsMandanas GabrielNo ratings yet
- Ores and Metallurgy-03-Assignments (New)Document13 pagesOres and Metallurgy-03-Assignments (New)Raju SinghNo ratings yet
- Chemical Formula Writing Worksheet2Document2 pagesChemical Formula Writing Worksheet2عابدهعلي100% (1)
- More Extra Nomenclature Practice - KEYDocument10 pagesMore Extra Nomenclature Practice - KEYelitzelmartinez21No ratings yet
- Namingpacketanswers 3Document14 pagesNamingpacketanswers 3Supremo DelagerNo ratings yet
- WKS001 010 636149 PDFDocument2 pagesWKS001 010 636149 PDFjulsNo ratings yet
- JEE ChemistryDocument406 pagesJEE Chemistryak1740120No ratings yet
- C Ch-16 General+Principles+and+Processes+OfDocument3 pagesC Ch-16 General+Principles+and+Processes+Ofmysoftinfo.incNo ratings yet
- Non-Metals Multiple Choice (CXC) PDFDocument3 pagesNon-Metals Multiple Choice (CXC) PDFjael SupervilleNo ratings yet
- 2.4.3 Chemical Formula and Naming Practice QuestionsDocument7 pages2.4.3 Chemical Formula and Naming Practice Questionsphat.vuongNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice Worksheetarlene serdiniaNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetSunshine LadyNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetOlivia DitzelNo ratings yet
- Word EquationsDocument6 pagesWord EquationsJohnRenzoMolinarNo ratings yet
- Tma 5Document12 pagesTma 5Rosa WellsNo ratings yet
- Lab Report #4Document11 pagesLab Report #4markNo ratings yet
- 9701 w09 QP 11Document16 pages9701 w09 QP 11Hubbak KhanNo ratings yet
- 0620 s09 QP 1Document32 pages0620 s09 QP 1h7h8No ratings yet
- CDS CubicDocument2 pagesCDS CubicSaRaii CalveeloNo ratings yet
- Acidic Nature of Organic CompoundsDocument5 pagesAcidic Nature of Organic CompoundsSakshi GargNo ratings yet
- Study Material Class 10 Chapter 2 2017 PDFDocument12 pagesStudy Material Class 10 Chapter 2 2017 PDFKaran Pratap100% (3)
- Board ProceedingsDocument432 pagesBoard Proceedingsdeepak kumarNo ratings yet
- Crystallography CompoundsDocument19 pagesCrystallography CompoundsgabisaNo ratings yet
- 316L SA 240 Ferguson MetalDocument1 page316L SA 240 Ferguson MetalSugiarto SaptomoNo ratings yet
- MSDS PhenophetalinDocument6 pagesMSDS PhenophetalinJohan SonNo ratings yet
- SRL Catalogue 20 21 Excel Version 27-05-2020Document315 pagesSRL Catalogue 20 21 Excel Version 27-05-2020Vanshika JainNo ratings yet
- Learning Module in Science 7Document10 pagesLearning Module in Science 7Mae LleovitNo ratings yet
- A Treatise On Chemistry 2i PDFDocument511 pagesA Treatise On Chemistry 2i PDFluis peixotoNo ratings yet
- 3 Woda 2016 Woda 5 10062016Document14 pages3 Woda 2016 Woda 5 10062016AdrianoNo ratings yet
- Conversion of ACH To AlOHDocument15 pagesConversion of ACH To AlOHDodi AfandiNo ratings yet
- Water Lecture-3: Water For Industrial Use Scale & SludgeDocument23 pagesWater Lecture-3: Water For Industrial Use Scale & Sludgesayan halderNo ratings yet
- Pure Chem p2 - 26pgDocument26 pagesPure Chem p2 - 26pgJhomer CrespoNo ratings yet
- Hef Durferrit QPQ Process EngDocument20 pagesHef Durferrit QPQ Process EngDemon'sHeartNo ratings yet
- A2 Unit 4 Chap 2 Past PapersDocument18 pagesA2 Unit 4 Chap 2 Past PapersTayyaba Mumtaz KhanNo ratings yet
- 5070 s10 QP 12Document16 pages5070 s10 QP 12Ruby ChongNo ratings yet
- Manufacture of ACETARSOLDocument2 pagesManufacture of ACETARSOLjpb4618No ratings yet
- Class PPT 1Document27 pagesClass PPT 1Ankita SinghNo ratings yet
- Dilution of Sodium Carbonate With CalciumDocument9 pagesDilution of Sodium Carbonate With CalciumMuhammad HamzaNo ratings yet
- Sorbenty Termoksid Dlya Atomnoy Otrasli Angl - VersiyaDocument10 pagesSorbenty Termoksid Dlya Atomnoy Otrasli Angl - Versiyaandreinanu73No ratings yet