Professional Documents
Culture Documents
003 Previous Chemistry Class Chemistry Notes 03 2022-07-18
003 Previous Chemistry Class Chemistry Notes 03 2022-07-18
Uploaded by
SarvOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
003 Previous Chemistry Class Chemistry Notes 03 2022-07-18
003 Previous Chemistry Class Chemistry Notes 03 2022-07-18
Uploaded by
SarvCopyright:
Available Formats
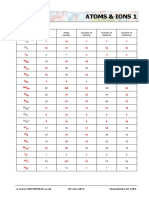
1
(Chemistry)
Atomic Number
7
'Z'
(Z) (P) (e)
Note:– 7 4
11 Na 23 92 U 235
z 11 z 92
Z P e
p 11 p 92
Na 23 Ca 40 Ar 40
11 20 18
e 11 e 92
z 11 z 20 z 18
A 23 A 235
p 11 p 20 p 18
n 23 – 1 22 n 235 92 143
e 11 e 20 e 18
(Ions) (Isobar)–
14 14
6C 7N
40 40
18Ar 20Ca
(Isotop)–
11Na 20Ca 17Cl –
1 2 3
z 11 z 20 z 17 1H 1 H 1 H
p 11 p 20 p 17 Polonium (Po) 27
e 10 e 18 e 17 1 18
Remark :-
(Isoelectronic)
mg e 12 2 10 (i)
12 1
e 13 – 3 10 1H – (n = 0)
13 Al 2
1H – (n = 1)
8O
––
e 8 2 10 3
1H – (n = 2)
(Atomic Mass) A (ii) 235 238
92U 92U
n = 143 n = 146
(iii) 12 14
6C 6C
= + n=6 n=8
A N P/Z A N Z
= – (i) –14 (C14)
N AZ
(ii) U235
Mob. : 8877918018, 8757354880 [By - Khan SIR ]
KHAN G. S. RESEARCH CENTRE 2
(iii) I131 Spdf
(iv) Fe59 S
(v) As74
(vi) Co60
(vii) Na23
Na24 S
P
(ISO-TONES)–
(Iso-tones)
(i) 14 16
6C 8O
n=8 n=8
p
(ii) 1H3 4 d
2He
n=2 n=2
(iii) 15P31 16S
32
n = 16 n = 16
(Orbit) (Shell)–
f
(Orbit)
K, L, M, N.....
K–
Remark:-
2n2 n
(ORBITS)
K n=1 e = 2n2 2 12 = 2
L n=2 e = 2n2 2 22 = 8
M n=3 e = 2n2 2 32 = 18
N n=4 e = 2n2 2 42 = 32
(Sub-orbit / Sub-shell)–
Mob. : 8877918018, 8757354880 [By - Khan SIR ]
KHAN G. S. RESEARCH CENTRE 3
6C = 1s2, 2s2, 2p2
Principle
(i) 24Cr 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d4
p
1s2, 2s2, 2p6, 3s2, 3p6, 3d5, 4s1
p d or [Ar] 3d5, 4s
p d f
(ii) 29Cu 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d9
1s2, 2s2, 2p6, 3s2, 3p6, 4s1, 3d10
(a) 7 [Ar] 4s1, 3d10
(iii) 79Au 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10,
(b) 7 4p6, 5s2, 4d10, 5p6, 6s2, 4f14, 5d9
1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10,
(c) 7 4p6, 5s2, 4d10, 5p6, 5d10, 4f14, 6s1
(iv) 47Ag 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10,
(d) 4 4s2, 3d10, 4p5
Note :- 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10,
4p6, 3d10, 4s1
–
(Quantum Number)
(Electronic Configuration)
Quantum Number
1s
1. Principal Quantum Number)
2p 2. (Azimuthal Quantum Number)
2s
3. (Magnetic Quantum Number)
3d 4. (Spin Quantum Number)
3p
3s 4f 1. Principal Quantum Number) :
4d
4p
4s
5d 5f Principal Quantum Number 'n' Denote
5p n = 1, 2, 3.
5s
Note :- Principal Quantum Number
6p 6d 6f
6s 2. (Azimuthal Quantum Number) :
Azimuthal Quantum Number "l" Denote
1
1H = 1s l = (n – 1)
2
2He = 1s s l=0
2 1
3Li = 1s , 2s p l=1
2 2
4Be = 1s , 2s d l=2
2 2 1
5B = 1s , 2s 2p f l=3
Mob. : 8877918018, 8757354880 [By - Khan SIR ]
KHAN G. S. RESEARCH CENTRE 4
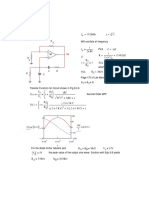
3. (Magnetic Quantum Number) : 3. n =3
'm' Denote l =5
m = – l to + l
m =
p l=1
m = –1, 0, +1 1
–1 0 +1 s
2
4. n =4
4. (Spin Quantum Number) :
l =2
s m =
Clock Wise ( =
1
Anti Clock Wise ( = s
2
2 He 10 Ne 18 Ar 36 Kr 54 Xe 86 Rn
1 1
S=+ S=–
2 2 2s 3s 4s 5s 6s 7s
Na 10 Ne 3s1
Q. 9F 1s2 2s2 2p5 electron 11
Sol. (n) = 2 12 Mg 10 Ne 3s2
(l) = 1
Remarks :- d- 4 9
–1 0 1 f 6 13
(m) = –1, 0, +1
1
(s) = =
2 Principal Quantum Number, Agimuthal Quantum
Anticlockwise direction Number, Magnetic Quantum Number
Q. Spine (Qn)
Sol. Na 1s2, 2s2, 2p6, 3s1
n =2 11 Na 1s2 2s2 2p6 3s1
l =p 10th electron 9th electron
m =
n2 n2
1 l 1 l 1
s
2 m 1, 0, 1 m 1, 0, 1
Q.
Sol. : Cl 1s2, 2s2, 2p6, 3s2 1 1
Cl 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10 s s
20 2 2
1. n =3
l =3
m =
1
s
2
2. n =3 h
l = 12 p
m =
h
h
1 mv
s
2
Mob. : 8877918018, 8757354880 [By - Khan SIR ]
You might also like
- Toyota ALPHARD VELLFIRE (EM2441E) - System Wiring Diagram PDFDocument910 pagesToyota ALPHARD VELLFIRE (EM2441E) - System Wiring Diagram PDFpengelana satu95% (20)
- Yanmar Part Catalog 4TNE-106T G1ADocument54 pagesYanmar Part Catalog 4TNE-106T G1ASemproel Beken100% (3)
- Roessler Table For Directional, Simple Paired Comparison and Duo TrioDocument1 pageRoessler Table For Directional, Simple Paired Comparison and Duo TrioKishoNo ratings yet
- اسئلة workshopDocument6 pagesاسئلة workshoprepel24564No ratings yet
- Ee Midsem SolutionDocument6 pagesEe Midsem SolutionTouch RosaNo ratings yet
- CH©VQ Mviwy: (X) (X) Y (Y + 10)Document11 pagesCH©VQ Mviwy: (X) (X) Y (Y + 10)ZamiNo ratings yet
- Ispulc Liy Cea, uCS22) Samikshalyng: Rven Als PubicDocument5 pagesIspulc Liy Cea, uCS22) Samikshalyng: Rven Als PubicssiNo ratings yet
- Steel Plates - Beams & Pipes RequirementsDocument3 pagesSteel Plates - Beams & Pipes RequirementsOmar WardehNo ratings yet
- Schematic ENC28J60 Ethernet Board 2022-12-14Document1 pageSchematic ENC28J60 Ethernet Board 2022-12-14samsularief03100% (1)
- Ece6950 1 - and 3-Phase ReviewDocument14 pagesEce6950 1 - and 3-Phase ReviewAhmed SabriNo ratings yet
- LOP Genset TegasDocument6 pagesLOP Genset TegasGOGO COllZZssNo ratings yet
- 7 HiluxDocument1 page7 HiluxautocomtrucksNo ratings yet
- JeppView - OBBI (19 Charts)Document23 pagesJeppView - OBBI (19 Charts)Seyi WilliamsNo ratings yet
- Lo Ind GM Be/2: - Oug SeumDocument7 pagesLo Ind GM Be/2: - Oug SeumAnanya PandaNo ratings yet
- HT 3Document45 pagesHT 3Mohammad SaqlainNo ratings yet
- Elec QuasarDocument30 pagesElec QuasarIH MedranoNo ratings yet
- IntefaceDocument1 pageIntefaceMedo O. EzzatNo ratings yet
- ULinkDocument1 pageULinkevandroNo ratings yet
- SPC AssignmentDocument5 pagesSPC AssignmentbhuvaneshNo ratings yet
- MT8870 Schematic DiagramDocument1 pageMT8870 Schematic DiagramAnonymous JoB5Zxg100% (1)
- Aoo) o - 1 O: Ncne-Ns Asba AhmedDocument5 pagesAoo) o - 1 O: Ncne-Ns Asba AhmedNusaiba AhmedNo ratings yet
- Adobe Scan 13 Oct 2021Document1 pageAdobe Scan 13 Oct 2021boobooNo ratings yet
- Ece1240 NC HWCMPDocument3 pagesEce1240 NC HWCMPJoel KimNo ratings yet
- RobotDocument1 pageRobotJyotirekha PatiNo ratings yet
- Paper A4Document30 pagesPaper A4Rohit AttriNo ratings yet
- Chemsheets Atoms and IonsDocument1 pageChemsheets Atoms and IonsTommy CuninghameNo ratings yet
- TV VOLTEL CY1429-001 LC8635xxx (N701)Document2 pagesTV VOLTEL CY1429-001 LC8635xxx (N701)Piscupescu DanNo ratings yet
- Q.rs L A. B: 6 Zinc 16 16Document13 pagesQ.rs L A. B: 6 Zinc 16 16Karigar DecorsNo ratings yet
- Limit Compr 110 Schematic 02Document1 pageLimit Compr 110 Schematic 02samoNo ratings yet
- FrameDocument22 pagesFramesgumble3007No ratings yet
- BEtc AssignmentDocument5 pagesBEtc AssignmentSoumyadip MoniNo ratings yet
- Hypothesis: Ho Ha - 10Document2 pagesHypothesis: Ho Ha - 10NeehaNo ratings yet
- 21mech007 Mithun.k.VDocument6 pages21mech007 Mithun.k.VMITHUN K VNo ratings yet
- ATMEGA328P-AU Breadboard PololuVersionDocument1 pageATMEGA328P-AU Breadboard PololuVersionSajad DehghanNo ratings yet
- Analog Electronics Assignment (Ee001-3-2-Ae)Document6 pagesAnalog Electronics Assignment (Ee001-3-2-Ae)maulana aji marwantoNo ratings yet
- Img 20230504 0004Document1 pageImg 20230504 0004sooriya kumarNo ratings yet
- Electron Structure WorksheetDocument1 pageElectron Structure WorksheetKirti KumarNo ratings yet
- 8035 Tme 1Document15 pages8035 Tme 1ayushkumarbhardwaj1562No ratings yet
- Chapter/Section Number Title Number Number: Ballast Installation Plate Installation, Leveling 08-99-00Document6 pagesChapter/Section Number Title Number Number: Ballast Installation Plate Installation, Leveling 08-99-00edward alba torresNo ratings yet
- Wien Bridge Oscillator PDFDocument22 pagesWien Bridge Oscillator PDFMohil koliNo ratings yet
- 60 Avensis (Cont. Next Page) : Charging (From May 2015 Production)Document2 pages60 Avensis (Cont. Next Page) : Charging (From May 2015 Production)zemzemi aliNo ratings yet
- Eis FDocument3 pagesEis Fforeman pemudaNo ratings yet
- Rvce Eee NotesDocument10 pagesRvce Eee NotesRahul ChandraNo ratings yet
- BK91-1318-01-FSF-000-PIP-RFI-0029 Request For Inspection of Carbon Steel Pipe Material - SignedDocument293 pagesBK91-1318-01-FSF-000-PIP-RFI-0029 Request For Inspection of Carbon Steel Pipe Material - SignedPanneer SelvamNo ratings yet
- Quiz2-ECE209 F09 SolnsDocument1 pageQuiz2-ECE209 F09 SolnsrosediodesNo ratings yet
- Tech BoardDocument1 pageTech BoardJason MatonisNo ratings yet
- 4-1 Component LocationDocument3 pages4-1 Component Locationwilfredo escobar gutierrezNo ratings yet
- 13 Hilux: Back-Up LightDocument1 page13 Hilux: Back-Up LightautocomtrucksNo ratings yet
- Fem 3Document3 pagesFem 3deepankarNo ratings yet
- Adobe Scan 15-Jan-2023Document25 pagesAdobe Scan 15-Jan-2023Ira ChaudhariNo ratings yet
- Hin To - Detesmine U Teility Dhe Saito3: RED Exot-4Document3 pagesHin To - Detesmine U Teility Dhe Saito3: RED Exot-4Shubh ShahNo ratings yet
- Onsm S A0001039453 1Document8 pagesOnsm S A0001039453 1Irvin Adan Lujan HernandezNo ratings yet
- MOD-LCD2.8RTP RevBDocument1 pageMOD-LCD2.8RTP RevBsureshotstudioNo ratings yet
- 2TLC010007T0102 Connection Example JSBRT11 To SSR10Document1 page2TLC010007T0102 Connection Example JSBRT11 To SSR10Bon BencavNo ratings yet
- Drawing4 ModelDocument1 pageDrawing4 Modelgenave7744No ratings yet
- GC-20 SchematicDocument1 pageGC-20 SchematicPetr BruzaNo ratings yet
- PIC Micro Project Board PDFDocument1 pagePIC Micro Project Board PDFOkiPetrus Hutauruk LumbanBaringin100% (2)
- Relay BDDocument1 pageRelay BDgiatrispktNo ratings yet
- Ecg 2Document1 pageEcg 2Hafizh Ammar WahyudiNo ratings yet