Professional Documents
Culture Documents
C Ch-20 Organic+Chemistry
Uploaded by
AKSHAYADITYA K RCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
C Ch-20 Organic+Chemistry
Uploaded by
AKSHAYADITYA K RCopyright:
Available Formats
Chapter 20
Organic Chemistry :
Some Basic Principles and Techniques
Only One Option Correct Type Questions
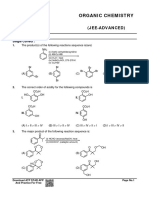
1. The number of structural isomers for C6H14 is [IIT-JEE-2007 (Paper-1)]
(A) 3 (B) 4
(C) 5 (D) 6
2. Among the following, the least stable resonance structure is [IIT-JEE-2007 (Paper-2)]
O O
(A) N (B) N
| |
O O
O O
(C)
N (D)
N
| |
O O
3. Sodium fusion extract, obtained from aniline, on treatment with iron (II) sulphate and H2SO4 in presence of air gives
a Prussian blue precipitate. The blue colour is due to the formation of [IIT-JEE-2007 (Paper-2)]
(A) Fe4[Fe(CN)6]3 (B) Fe3[Fe(CN)6]2
(C) Fe4[Fe(CN)6]2 (D) Fe3[Fe(CN)6]3
4. Hyperconjugation involves overlap of the following orbitals [IIT-JEE-2008 (Paper-1)]
(A) – (B) –p
(C) p – p (D) –
5. The correct stability order for the following species is [IIT-JEE-2008 (Paper-2)]
I.
O
II.
III.
O
IV.
(A) (II) > (IV) > (I) > (III) (B) (I) > (II) > (III) > (IV)
(C) (II) > (I) > (IV) > (III) (D) (I) > (III) > (II) > (IV)
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005 Ph.011-47623456
ARCHIVE - JEE (Advanced) CHEMISTRY
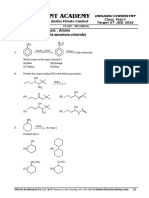
6. The correct acidity order of the following is [IIT-JEE-2009 (Paper-1)]
OH OH COOH COOH
Cl CH3
(I) (II) (III) (IV)
(A) (III) > (IV) > (II) > (I) (B) (IV) > (III) > (I) > (II)
(C) (III) > (II) > (I) > (IV) (D) (II) > (III) > (IV) > (I)
7. The IUPAC name of the following compound is [IIT-JEE-2009 (Paper-1)]
OH
CN
Br
(A) 4-Bromo-3-cyanophenol (B) 2-Bromo-5-hydroxybenzonitrile
(C) 2-Cyano-4-hydroxybromobenzene (D) 6-Bromo-3-hydroxybenzonitrile
8. In the following carbocation, H/CH3 that is most likely to migrate to the positively charged carbon is
[IIT-JEE-2009 (Paper-2)]
H H
1 + 5
2 4
H3C—C —C—C
3
—CH3
HO H CH3
(A) CH3 at C-4 (B) H at C-4
(C) CH3 at C-2 (D) H at C-2
9. The correct stability order of the following resonance structures is [IIT-JEE-2009 (Paper-2)]
– –
I. H2C N N II. H2 C— N N
– –
III. H2 C— N N IV. H2 C— N N
(A) (I) > (II) > (IV) > (III) (B) (I) > (III) > (II) > (IV)
(C) (II) > (I) > (III) > (IV) (D) (III) > (I) > (IV) > (II)
10. Which one of the following structures has the IUPAC name 3-ethynyl-2-hydroxy-4-methylhex-3-en-5-ynoic acid?
[IIT-JEE-2020 (Paper-1)]
OH
(A) CO 2H (B) HO 2C
OH
OH OH
(C) CO 2H (D) CO 2H
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005 Ph.011-47623456
CHEMISTRY ARCHIVE - JEE (Advanced)
11. Among the following, the conformation that corresponds to the most stable conformation of meso-butane-2,3-
diol is [JEE (Adv)-2021 (Paper-1)]
OH OH
H Me H OH
(A) (B)
Me H H Me
OH Me
OH OH
Me OH H OH
(C) (D)
H Me Me H
H Me
One or More Option(s) Correct Type Questions
12. The correct statement(s) concerning the structures E, F and G is (are) [IIT-JEE-2008 (Paper-1)]
O H3C OH H3C CH3
H3C
H3C CH3 H3C CH3 H3C OH
(E) (F) (G)
(A) E, F and G are resonance structures
(B) E, F and E, G are tautomers
(C) F and G are geometrical isomers
(D) F and G are diastereomers
13. Amongst the given options the compound(s) in which all the atoms are in one plane in all the possible
conformations (if any), is(are) [IIT-JEE-2011 (Paper-1)]
H H H
(A) C—C (B) H—C C—C
H 2C CH2 CH2
(C) H2C = C = O (D) H2C = C = CH2
14. Which of the following molecules, in pure form, is (are) unstable at room temperature? [IIT-JEE-2012 (Paper-1)]
(A) (B)
O
O
(C) (D)
15. The hyperconjugative stabilities of tert-butyl cation and 2-butene, respectively, are due to [JEE (Adv)-2013 (Paper-1)]
(A) p (empty) and electron delocalisations
(B) * and electron delocalisations
(C) p (filled) and electron delocalisations
(D) p (filled) and electron delocalisations
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005 Ph.011-47623456
ARCHIVE - JEE (Advanced) CHEMISTRY
16. The IUPAC name(s) of the following compound is(are) [JEE (Adv)-2017 (Paper-1)]
H3C Cl
(A) 1-chloro-4-methylbenzene (B) 4-chlorotoluene
(C) 1-methyl-4-chlorobenzene (D) 4-methylchlorobenzene
17. With respect to the compounds I-V, choose the correct statement(s). JEE (Adv)-2020 (Paper-1)]
H
H CH3
I II III
H H
IV V
(A) The acidity of compound I is due to delocalization in the conjugate base
(B) The conjugate base of compound IV is aromatic
(C) Compound II becomes more acidic, when it has a -NO2 substituent
(D) The acidity of compounds follows the order I > IV > V > II > III
Integer / Numerical Value Type Questions
18. The total number of cyclic structural as well as stereoisomers possible for a compound with the molecular
formula C5H10 is [IIT-JEE-2009 (Paper-2)]
19. The total number of cyclic isomers possible for a hydrocarbon with the molecular formula C4H6 is
[IIT-JEE-2010 (Paper-1)]
20. The total number of contributing structures showing hyperconjugation (involving C-H bonds) for the following
carbocation is [IIT-JEE-2011 (Paper-2)]
H3C CH2CH3
+
21. The number of resonance structures for N is [JEE (Adv)-2015 (Paper-1)]
OH
NaOH
N
22. Among the following, the number of aromatic compound(s) is [JEE (Adv)-2017 (Paper-1)]
+ +
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005 Ph.011-47623456
You might also like
- Solution Manual for The Elements of Polymer Science and EngineeringFrom EverandSolution Manual for The Elements of Polymer Science and EngineeringRating: 4 out of 5 stars4/5 (3)
- Sample Acs Final ExamDocument27 pagesSample Acs Final Examjilo100% (2)
- Chapter 5: Structure and Preparation of Alkenes - Elimination ReactionsDocument13 pagesChapter 5: Structure and Preparation of Alkenes - Elimination ReactionsRahma AshrafNo ratings yet
- JEE Main 2019 Paper Answer Chemistry 09-01-2019 1stDocument6 pagesJEE Main 2019 Paper Answer Chemistry 09-01-2019 1stDeepak SainiNo ratings yet
- General Organic Chemistry ProblemsDocument14 pagesGeneral Organic Chemistry ProblemsHarsh MeenaNo ratings yet
- Vibrant Academy: (India) Private LimitedDocument6 pagesVibrant Academy: (India) Private LimitedRk ChaudharyNo ratings yet
- C Ch-18 Co-Ordination CompoundsDocument7 pagesC Ch-18 Co-Ordination Compoundsmysoftinfo.incNo ratings yet
- Aziridinone Azetididone Buatanamide: Ome Pocl Reflux, 2HDocument2 pagesAziridinone Azetididone Buatanamide: Ome Pocl Reflux, 2HAllu HarikrishnaNo ratings yet
- .J - S IR: Alcohol, Ether & PhenolDocument6 pages.J - S IR: Alcohol, Ether & PhenolPreetam PaulNo ratings yet
- Reactive Intermediate TestDocument6 pagesReactive Intermediate TestDhruv patelNo ratings yet
- Organic chemistry principles and techniques testDocument6 pagesOrganic chemistry principles and techniques testishitaNo ratings yet
- AromaticDocument29 pagesAromaticgoswamiaddNo ratings yet
- C - Ch-24 - Aldehydes, Ketones and Carboxylic AcidsDocument22 pagesC - Ch-24 - Aldehydes, Ketones and Carboxylic Acidsmysoftinfo.incNo ratings yet
- IIT-JAM 2016 With SolutionDocument25 pagesIIT-JAM 2016 With SolutiongauravNo ratings yet
- 04 Hydrocarbon Set Test Final EDocument3 pages04 Hydrocarbon Set Test Final EΒίητ ε ΗάωωάNo ratings yet
- Mixed DPP 8 - 20 (Isomerism)Document37 pagesMixed DPP 8 - 20 (Isomerism)shresthgaur19No ratings yet
- Goc Combined Iit Jam Cemistry QuestionsDocument15 pagesGoc Combined Iit Jam Cemistry QuestionsSandipan Saha67% (3)
- Name The Following Compounds According To IUPAC System of Nomenclature (Document2 pagesName The Following Compounds According To IUPAC System of Nomenclature (AbubakarNo ratings yet
- JEE ORGANIC CHEMISTRY REVIEWDocument5 pagesJEE ORGANIC CHEMISTRY REVIEWDrushya SalunkeNo ratings yet
- Quiz-General Organic Chemistry & Isomerism-Snd - SNDDocument4 pagesQuiz-General Organic Chemistry & Isomerism-Snd - SNDayesha sheikhNo ratings yet
- Alkyl Halide ReactionsDocument3 pagesAlkyl Halide ReactionsKryonNo ratings yet
- Exercise - VI (A) : IIT-JEE (Objective Problems)Document3 pagesExercise - VI (A) : IIT-JEE (Objective Problems)MoneyNo ratings yet
- Jumbo Test-2Document5 pagesJumbo Test-2prithvijeetopNo ratings yet
- BocheemistryDocument9 pagesBocheemistryponveeraventhanpNo ratings yet
- For More Material Join: @jeeadvanced - 2024: Level 2Document47 pagesFor More Material Join: @jeeadvanced - 2024: Level 2anuragrana12345678No ratings yet
- Reaction IntermediatesDocument32 pagesReaction Intermediatestechno studioNo ratings yet
- PS # 18 Coordination CompoundDocument2 pagesPS # 18 Coordination CompoundanirudhsingotyiaNo ratings yet
- Goc-II Ex e SiamrpnDocument22 pagesGoc-II Ex e SiamrpnArchit KhemaniNo ratings yet
- Alcohol, Ether, Phenol WorksheetDocument22 pagesAlcohol, Ether, Phenol WorksheetSDMNo ratings yet
- GOC (13th)Document34 pagesGOC (13th)Raju SinghNo ratings yet
- Atp Mega RevDocument14 pagesAtp Mega RevSURAKSHA PATELNo ratings yet
- C - Ch-15 - The P-Block Elements (Group 13 To Group 18)Document7 pagesC - Ch-15 - The P-Block Elements (Group 13 To Group 18)mysoftinfo.incNo ratings yet
- M.M. : 35 DPP # 04 TIME : 30 MINDocument2 pagesM.M. : 35 DPP # 04 TIME : 30 MINArjun SabnisNo ratings yet
- Main Group Elements NET/JRF Previous Years' Question SummaryDocument19 pagesMain Group Elements NET/JRF Previous Years' Question SummaryNaman AroraNo ratings yet
- GOC - DPP 02 - Yakeen 2.0 2024 (Legend)Document3 pagesGOC - DPP 02 - Yakeen 2.0 2024 (Legend)bandarbarfilaNo ratings yet
- Chemistry - Mains1 (Entire 11th)Document9 pagesChemistry - Mains1 (Entire 11th)Ravi Kiran KoduriNo ratings yet
- Allen DPP1 Hydrocarbons PDFDocument6 pagesAllen DPP1 Hydrocarbons PDFJannaki PvNo ratings yet
- Jumbo Home Test-1 - FinalDocument59 pagesJumbo Home Test-1 - FinalSayali SachinNo ratings yet
- Spotlight_Phase_2_2021_22_Day_1_In_Class_Assingement_Chemistry_OnlyDocument8 pagesSpotlight_Phase_2_2021_22_Day_1_In_Class_Assingement_Chemistry_Onlysnohkmr04136No ratings yet
- PRACTICE SHEET - 03 (Chemistry) : CH - CH - CH CN CN CNDocument3 pagesPRACTICE SHEET - 03 (Chemistry) : CH - CH - CH CN CN CNABD 17No ratings yet
- Organic Chemistry: Daily Practice ProblemsDocument10 pagesOrganic Chemistry: Daily Practice ProblemsRaju SinghNo ratings yet
- C Ch-25 AminesDocument7 pagesC Ch-25 Aminesmysoftinfo.incNo ratings yet
- First Year - GOC, ISOMERISM - Revision - CPP - CKHDocument3 pagesFirst Year - GOC, ISOMERISM - Revision - CPP - CKHSeaN GabrielNo ratings yet
- Alcohol, Phenol & EtheRDocument8 pagesAlcohol, Phenol & EtheRdevvratchoudhary11989No ratings yet
- Iupac 1Document37 pagesIupac 1shodhan shettyNo ratings yet
- Previous Years Iit - Jee Questions: General Organic ChemistryDocument8 pagesPrevious Years Iit - Jee Questions: General Organic ChemistryudaysrinivasNo ratings yet
- Class Test-1_Amine (Benzene-dia-azonium chloride)_WithoutDocument8 pagesClass Test-1_Amine (Benzene-dia-azonium chloride)_WithoutshouryatrialNo ratings yet
- DPP 4Document2 pagesDPP 4sumitNo ratings yet
- SECTION-I (Single Choice Questions) : IIT - JEE: 2016 Crash Course (C 7 - A 1) Date: Topic: Iupac, Nomenclature, GocDocument11 pagesSECTION-I (Single Choice Questions) : IIT - JEE: 2016 Crash Course (C 7 - A 1) Date: Topic: Iupac, Nomenclature, GocSachin DedhiaNo ratings yet
- Chemical Bonding - Practice Sheet - (NSEC)Document3 pagesChemical Bonding - Practice Sheet - (NSEC)aryanNo ratings yet
- Adv. Prev + Extra Edge 68-74 (Exercise 5 & 6)Document7 pagesAdv. Prev + Extra Edge 68-74 (Exercise 5 & 6)Aditya ShahNo ratings yet
- Stereoisomerism Exercise PDFDocument51 pagesStereoisomerism Exercise PDFGOURISH AGRAWAL100% (3)
- FIITJEE JEE MAIN TEST SERIES PAPERDocument20 pagesFIITJEE JEE MAIN TEST SERIES PAPERsanjeev1000No ratings yet
- Target JEE 2022 Chemistry Practice ExamDocument13 pagesTarget JEE 2022 Chemistry Practice ExamKritikaNo ratings yet
- GOC - Acid - Base QDocument11 pagesGOC - Acid - Base Qphokatka0No ratings yet
- Acidic Strength-Basic StrengthDocument19 pagesAcidic Strength-Basic Strengthchessguy2847No ratings yet
- Mock Test 6 P 1 Bks CDocument18 pagesMock Test 6 P 1 Bks CRare RootNo ratings yet
- @bohring - Bot - GOC, Isomerism & EAS @HeyitsyashXDDocument5 pages@bohring - Bot - GOC, Isomerism & EAS @HeyitsyashXDxkryxxzNo ratings yet
- Co-Ordination Compounds - PYQ - (NSEC)Document4 pagesCo-Ordination Compounds - PYQ - (NSEC)aryanNo ratings yet
- 016) Main GroupDocument31 pages016) Main GroupHarish RamachandraNo ratings yet
- Ans-Sol JEEMain-2022 Phase-2!26!07-2022 M Chemistry FINALDocument7 pagesAns-Sol JEEMain-2022 Phase-2!26!07-2022 M Chemistry FINALryarpit0No ratings yet
- IR Dry LabDocument17 pagesIR Dry LabhimaNo ratings yet
- Reaction IntermediatesDocument42 pagesReaction IntermediatestareNo ratings yet
- 2008 Tadross2Document98 pages2008 Tadross2potpuraaaNo ratings yet
- Characterization of Lignin Extracted CompoundDocument13 pagesCharacterization of Lignin Extracted CompoundJitender SinghNo ratings yet
- 17 de Thi Hoc Ki 1 Mon Hoa Hoc Lop 10Document379 pages17 de Thi Hoc Ki 1 Mon Hoa Hoc Lop 10cclatrumNo ratings yet
- Aldehydes & Ketones: (Alkanals & Alkanones)Document21 pagesAldehydes & Ketones: (Alkanals & Alkanones)Firgin DeisyellaNo ratings yet
- PDF LambertiDocument8 pagesPDF LambertiFernanda Amaral FariaNo ratings yet
- Fat Facts PDFDocument6 pagesFat Facts PDFEko Ariyanto Wong PlembangNo ratings yet
- Aldehydes Ketones and Carboxylic Acids YuvabrigadeDocument4 pagesAldehydes Ketones and Carboxylic Acids YuvabrigadeRavishankar H SNo ratings yet
- BSc Molecular Biotechnology Program OverviewDocument7 pagesBSc Molecular Biotechnology Program Overviewjazlyn aceroNo ratings yet
- Charge-Transfer Complexes Between Iodine and Substituted Thioureas: Determination of Thermodynamic and Spectroscopic PropertiesDocument7 pagesCharge-Transfer Complexes Between Iodine and Substituted Thioureas: Determination of Thermodynamic and Spectroscopic PropertiesBob RoonyNo ratings yet
- Explore Molecular Shapes with PhET SimulationDocument7 pagesExplore Molecular Shapes with PhET SimulationEVAN BLIZZARDNo ratings yet
- Organic Chemistry Chapter Wise Previous Year QuestionDocument13 pagesOrganic Chemistry Chapter Wise Previous Year QuestionSachithNo ratings yet
- Polymers: Lecture 3 Unit-2b: The Mechanism of Addition PolymerizationDocument3 pagesPolymers: Lecture 3 Unit-2b: The Mechanism of Addition PolymerizationUtkarsh SinghNo ratings yet
- Berry FlorDocument5 pagesBerry FlorUST College of Science Glee ClubNo ratings yet
- Honeywell Gas Detectction System CalibrationDocument152 pagesHoneywell Gas Detectction System CalibrationShanmugamoorthyNo ratings yet
- Carbohydrate StructureDocument12 pagesCarbohydrate StructureAilyn DulzaNo ratings yet
- PGD-IRI Question Paper 2015Document4 pagesPGD-IRI Question Paper 2015Divasasi SasiNo ratings yet
- Nomenklatura Organskih SpojevaDocument13 pagesNomenklatura Organskih Spojevaplaninka_jaksic4160No ratings yet
- Presentation1 Degradation Mechanism of RubberDocument33 pagesPresentation1 Degradation Mechanism of Rubbersangleminh36No ratings yet
- Metal Organic Frameworks as Catalysts for Fine Chemical ProductionDocument32 pagesMetal Organic Frameworks as Catalysts for Fine Chemical Productionkarthiche05No ratings yet
- MassSpectroscopy - Rule of 13Document45 pagesMassSpectroscopy - Rule of 13Maxi Ma100% (1)
- Microwave-Assisted Extraction and Synthesis of Bioactive CompoundsDocument50 pagesMicrowave-Assisted Extraction and Synthesis of Bioactive Compoundsprashant mande100% (1)
- Mechanism of PolymeristionDocument25 pagesMechanism of PolymeristionAshish Kumar SinghNo ratings yet
- PH CH 126.1 Fischer Esterification of Methyl Benzoate 2Document6 pagesPH CH 126.1 Fischer Esterification of Methyl Benzoate 2Tammy CacnioNo ratings yet
- Haloalkanes & HaloarenesDocument28 pagesHaloalkanes & HaloarenesFam IlyNo ratings yet
- CH 1Document44 pagesCH 1angelita072No ratings yet
- Naproxen Derivatives Synthesis, Reactions 2017Document28 pagesNaproxen Derivatives Synthesis, Reactions 2017Luis EnriqueNo ratings yet
- Chem. Rev. 2009, 109, 5069-5119Document51 pagesChem. Rev. 2009, 109, 5069-5119dhuglNo ratings yet