Professional Documents
Culture Documents
SCC Tongue in Epidermolysis Simplex Bullosa
Uploaded by
Ali FaridiOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
SCC Tongue in Epidermolysis Simplex Bullosa
Uploaded by
Ali FaridiCopyright:
Available Formats
ONLINE CASE REPORT
Ann R Coll Surg Engl 2021; 103: e85–e87
doi 10.1308/rcsann.2020.7080
Locally advanced tongue squamous cell carcinoma
in epidermolysis simplex bullosa patient:
a therapeutic conundrum
W Al Shareef, S Sayed, S Kamel, H Alkaf, A Bahaj, R Amin, A Al Herabi
Head and Neck & Skull Base Centre, King Abdullah Medical City, Mecca, Saudi Arabia
ABSTRACT
Epidermolysis bullosa simplex (EBS) is a debilitating condition affecting the skin and mucous membranes that is characterised by frequent ulceration and
blistering on trivial trauma. In EBS, oral cavity mucosal injuries lead to a high propensity for developing squamous cell carcinomas. Locally advanced
tongue carcinoma arising in this background presents a challenging therapeutic conundrum. To our knowledge, this is the first case of aggressive
locally advanced tongue carcinoma that has developed sporadically in a patient with EBS and no family history. Routine screening of oral mucosal
lesions will lead to early detection and timely management of this debilitating condition.
KEYWORDS
Epidermolysis bullosa – Epidermolysis simplex bullosa – Oral cancer – Squamous cell carcinoma
– Tongue cancer
Accepted 20 October 2020
CORRESPONDENCE TO
Suhail Sayed, E: drsuhailsayed@gmail.com
Case history nonsurgical therapeutic options were discussed. The
patient opted for radiotherapy and received 30Gy/15
A 42-year-old man presented to our head and neck fractions of intensity modulated radiotherapy; treatment
oncology clinic with a history of non-healing tongue was discontinued as the patient developed grade III
mass. The patient also gave an account of dry, thin skin mucositis and grade IV skin toxicity (Radiation Therapy
that ‘blisters on minimal injury’. There was a history of Oncology Group classification; ulceration, haemorrhage
surgery for squamous cell carcinoma over the dorsum of and necrosis). Radiation therapy was discontinued in the
the right hand; wide excision and split-thickness skin wake of the severe toxicity. Subsequently, the patient was
grafting was done. There was no family history of similar given best supportive care, which included pain
illness nor any consanguinity. Similarly, there was no management and nutritional support through a feeding
history of abuse of tobacco or tobacco-related gastrostomy. The patient died due to disease progression
substances. Clinical examination revealed multiple four months after completing radiotherapy.
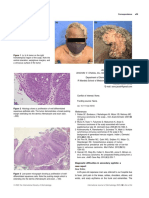
blisters and scarring on the extremities (Figure 1a,b),
extensive, widespread brownish reticulated patches over
the chest and neck (Figure 1c), restricted mouth opening,
oral commissure weakness, ankyloglossia and a 4 × 3cm2
Discussion
ulceroinfiltrative lesion involving the left middle This was a unique situation in which we needed to treat a
one-third oral tongue extending posterior to the tongue locally advanced stage IVa tongue carcinoma with an
base (Figure 1d). Magnetic resonance imaging (MRI) underlying debilitating skin condition, which was
revealed a 5 × 4cm2 lesion involving the left half and base characterised by the development of blisters and
of the tongue, extending along the left lingual ulcerations in the skin and mucous membranes on
neurovascular bundle and metastatic left, level IIa nodes minimal injury.1 The tongue is a mobile tissue, which
(Figure 1e). There was no evidence of distant metastasis makes it more liable to frequent dental trauma and
on preliminary evaluation. Tongue biopsy was suggestive undifferentiated cellular transformation leading to
of invasive well differentiated squamous carcinoma and squamous cell carcinoma.2 The patient’s condition
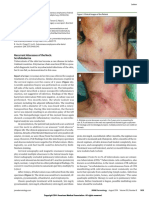
the skin biopsy on histology (haematoxylin and eosin) warranted a near-total glossectomy with neck dissection
revealed features of epidermolysis bullosa simplex (EBS), and soft tissue reconstruction (microvascular/pedicle
ie subepidermal noninflammatory blister and pale basal flap) followed by adjuvant chemoradiation.3 The surgical
keratinocytes (Figure 2a–d). During the multidisciplinary option seemed to be the most appropriate first-line
tumour board meeting, different surgical and treatment as the tumour was resectable. The major
Ann R Coll Surg Engl 2021; 103: e85–e87 e85
AL SHAREEF SAYED KAMEL ALKAF BAHAJ AMIN AL HERABI LOCALLY ADVANCED TONGUE SQUAMOUS CELL CARCINOMA
Figure 1 (a–c) Multiple blisters and scarring on the palms and soles with extensive, widespread brownish reticulated patches over the chest and
neck. (d) Oral cavity findings. (e) Magnetic resonance imaging scan T1W with contrast.
questions in front of the tumour board were: (i) morbidity severe systemic toxicity due to chemotherapy and
and quality of life post-surgery, (ii) success of radiation in patients with EBS.5 The current literature
reconstructive surgery, (iii) feasibility of adjuvant lacks substantial information related to the management
chemoradiation, and (iv) survival outcomes. Although of oral cavity cancers that arise in the background of
guidelines exist in the current literature for managing EBS.2,3 Although new therapeutics like immunotherapy
cutaneous squamous cell carcinomas in EBS, there is no and targeted therapy are still being evaluated in this
robust literature for managing oral cavity cancers arising patient subset, there is a need to design studies specific
in patients with EBS.4 As contemplated, the surgery would for oral mucosal squamous cell carcinomas.6,7
be morbid, and the patient would be dependent on
tracheostomy and gastrostomy feeding, significantly
affecting his quality of life. The chances of surgical wound
dehiscence/breakdown were substantial taking into
Conclusion
consideration the disease characteristics, ie skin fragility EBS is a debilitating skin condition. It can be challenging to
and desquamation. Furthermore, if the patient had manage if it presents with mucosal squamous cell
tolerated surgery, the course of adjuvant chemoradiation carcinomas. Because of the dearth of literature and no
would be more challenging, with the literature suggesting clear therapeutic protocols, there is a need for active
e86 Ann R Coll Surg Engl 2021; 103: e85–e87
AL SHAREEF SAYED KAMEL ALKAF BAHAJ AMIN AL HERABI LOCALLY ADVANCED TONGUE SQUAMOUS CELL CARCINOMA
Figure 2 (a–c) Epidermolysis bullosa simplex: A, noninflamed subepidermal blister; B, pallor of basilar keratinocytes (‘eraser effect’) (black arrow).
(d) Tongue biopsy shows irregular infiltrating islands of neoplastic keratinocytes typical of invasive well-differentiated squamous cell carcinoma.
surveillance of this category of patients for early detection 3. Montaudié H, Chiaverini C, Sbidian E et al. Inherited epidermolysis bullosa and
squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis
of any suspicious lesions of the oral mucosa. This will be
2016; 11: 117. Published 2016 Aug 20.
worthwhile in improving therapeutic outcomes and 4. Mellerio JE, Robertson SJ, Bernardis C et al. Management of cutaneous squamous cell
quality of life in this patient subset. carcinoma in patients with epidermolysis bullosa - best clinical practice guidelines. Br J
Dermatol 2016; 174: 56–67.
5. Bastin KT, Steeves RA, Richards MJ. Radiation therapy for squamous cell carcinoma in
dystrophic epidermolysis bullosa: case reports and literature review. Am J Clin Oncol
References
1997; 20: 55–58.
1. Lanschuetzer CM. Definition. In: Hintner H. Life with Epidermolysis Bullosa (EB)
6. Foote MC, McGrath M, Guminski A et al. Phase II study of single agent panitumumab in
Etiology, Diagnosis, Multidisciplinary Care and Therapy. New York: Springer; 2009.
patients with incurable cutaneous squamous cell carcinoma. Ann Oncol 2014; 25:
pp. 3–5.
2047–2052.
2. Baek JO, Lee HY, Oh SW et al. A novel homozygous keratin 14 mutation in a patient
7. El-Darouti M, Fawzy M, Amin I et al. Treatment of dystrophic epidermolysis bullosa with
with autosomal recessive epidermolysis bullosa simplex and squamous cell
carcinoma of the tongue. Br J Dermatol 2010; 162: 880–882. [published bone marrow non-hematopoeitic stem cells: A randomized controlled trial. Dermatol
correction appears in Br J Dermatol. 2010 Jun;162(6):1416]. Ther 2016; 29: 96–100.
Ann R Coll Surg Engl 2021; 103: e85–e87 e87
You might also like
- Namin 2005Document4 pagesNamin 2005Mohammed FaroukNo ratings yet
- Oral Cancer in A 5-Year-Old Boy: A Rare Case Report and Review of LiteratureDocument10 pagesOral Cancer in A 5-Year-Old Boy: A Rare Case Report and Review of LiteratureLidia Muñoz CastroNo ratings yet
- Ameloblastic Fibrosarcoma or Odontogenic Carcinosarcoma: A Matter of Classification?Document6 pagesAmeloblastic Fibrosarcoma or Odontogenic Carcinosarcoma: A Matter of Classification?Jeyachandran MariappanNo ratings yet
- Linfoma Extranodal en Cabeaza y CuelloDocument5 pagesLinfoma Extranodal en Cabeaza y CuelloCARLOS DARIO MERINO BUSTAMANTENo ratings yet
- Verrucous Carcinoma Ackerman'sDocument3 pagesVerrucous Carcinoma Ackerman'sdarsunaddictedNo ratings yet
- Angiomyolipoma of The Hard Palate: Clinical ReportDocument2 pagesAngiomyolipoma of The Hard Palate: Clinical ReportKezia Rachellea MustakimNo ratings yet
- Int J Dermatology - 2022 - Iwasawa - Diagnostic Difficulties in Secondary Syphilis A Case ReportDocument3 pagesInt J Dermatology - 2022 - Iwasawa - Diagnostic Difficulties in Secondary Syphilis A Case ReportArmando NogueraNo ratings yet
- Case Report: Palatal Swelling: A Diagnostic EnigmaDocument6 pagesCase Report: Palatal Swelling: A Diagnostic EnigmaPrince AhmedNo ratings yet
- Gingival and Submandibular Lymph Node Metastasis of Sigmoid Colon AdenocarcinomaDocument4 pagesGingival and Submandibular Lymph Node Metastasis of Sigmoid Colon AdenocarcinomafitriahendricoNo ratings yet
- Mixoma 9Document4 pagesMixoma 9Nicolás MedinaNo ratings yet
- Ameloblastic Carcinoma - Report of A New Case, Literature Review, and Comparison To AmeloblastomaDocument7 pagesAmeloblastic Carcinoma - Report of A New Case, Literature Review, and Comparison To AmeloblastomaTayyaba RafiqNo ratings yet
- Spindle Cell Tumor in Oral Cavity: A Rare Case Report: Ase EportDocument4 pagesSpindle Cell Tumor in Oral Cavity: A Rare Case Report: Ase EportDeka Dharma PutraNo ratings yet
- Bilateral Odontogenic Keratocyst in A NonsyndromicDocument5 pagesBilateral Odontogenic Keratocyst in A NonsyndromicRocio CacñahuarayNo ratings yet
- Synchronous Squamous Cell Carcinoma of The Lip and Nasopharyngeal Carcinoma - A Rare Case Report.Document4 pagesSynchronous Squamous Cell Carcinoma of The Lip and Nasopharyngeal Carcinoma - A Rare Case Report.International Journal of Innovative Science and Research Technology100% (1)
- Orbital Metastasis of A Pediatric Nasopharyngeal Carcinoma A Rare Case ReportDocument4 pagesOrbital Metastasis of A Pediatric Nasopharyngeal Carcinoma A Rare Case ReportInternational Journal of Innovative Science and Research Technology100% (1)
- BBRC Vol 14 No 04 2021-03Document4 pagesBBRC Vol 14 No 04 2021-03Dr Sharique AliNo ratings yet
- Article 6 2 6Document9 pagesArticle 6 2 6Gabriel LazarNo ratings yet
- Mandibular Mets From Known Breast CaDocument2 pagesMandibular Mets From Known Breast Cacalustre2016No ratings yet
- Two Cases of Subungual Melanoma of The Thumb in SituDocument5 pagesTwo Cases of Subungual Melanoma of The Thumb in SituIJAR JOURNALNo ratings yet
- CRTC1 Ca MucoEpider 10Document6 pagesCRTC1 Ca MucoEpider 10DanielaNo ratings yet
- J Joms 2014 08 040Document7 pagesJ Joms 2014 08 040Gisela LalitaNo ratings yet
- Primary Ameloblastic Carcinoma of The Maxilla A CaDocument9 pagesPrimary Ameloblastic Carcinoma of The Maxilla A CaMuhammad Avicenna AdjiNo ratings yet
- Keratocystic Odontogenic Tumor - A Case Report and Review of LiteratureDocument4 pagesKeratocystic Odontogenic Tumor - A Case Report and Review of Literaturearbi123No ratings yet
- (03241750 - Acta Medica Bulgarica) Cutaneous Xanthogranuloma of The Face - An Uncommon Clinical Diagnosis and Challenging ReconstructionDocument3 pages(03241750 - Acta Medica Bulgarica) Cutaneous Xanthogranuloma of The Face - An Uncommon Clinical Diagnosis and Challenging ReconstructionTeodorNo ratings yet
- Ojsadmin, 1340Document4 pagesOjsadmin, 1340Dr NisrinNo ratings yet
- Osteosarcoma of The Jaw: Report of 3 Cases (Including The Rare Epithelioid Variant) With Review of LiteratureDocument10 pagesOsteosarcoma of The Jaw: Report of 3 Cases (Including The Rare Epithelioid Variant) With Review of LiteraturesTEVENNo ratings yet
- Odontogenickeratocyst Clinically Mimicking Osteomyelitis - A Rare Case ReportDocument4 pagesOdontogenickeratocyst Clinically Mimicking Osteomyelitis - A Rare Case ReportInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- A Rare Case of Zosteriform Cutaneous Metastasesfrom Squamous Cell Carcinoma of Hard PalateDocument4 pagesA Rare Case of Zosteriform Cutaneous Metastasesfrom Squamous Cell Carcinoma of Hard PalateShiva PNo ratings yet
- Khalid, 356Document4 pagesKhalid, 356Indah VitasariNo ratings yet
- Diagnostic and Therapeutic Pieges Before Clinicoradiological Elements Eviding A Mandibular AmeloblastomaDocument4 pagesDiagnostic and Therapeutic Pieges Before Clinicoradiological Elements Eviding A Mandibular AmeloblastomaAnjay MabarNo ratings yet
- Cervical Cellulitis and Mediastinitis Caused by Odontogenic Infections. Report of Two Cases and Review of LiteratureDocument6 pagesCervical Cellulitis and Mediastinitis Caused by Odontogenic Infections. Report of Two Cases and Review of LiteratureJC QuezadaNo ratings yet
- Clear Cell Odontogenic Carcinoma of The Mandible: A Treatment StrategyDocument5 pagesClear Cell Odontogenic Carcinoma of The Mandible: A Treatment StrategyMaría Fernanda EstévezNo ratings yet
- CA LS Bướu Nhiều Hốc - Multilocular Radiolucency in the Body of MandibleDocument4 pagesCA LS Bướu Nhiều Hốc - Multilocular Radiolucency in the Body of MandibleThành Luân NguyễnNo ratings yet
- Ijcmr 692 Jun 14Document2 pagesIjcmr 692 Jun 14Jeyachandran MariappanNo ratings yet
- Unusual Cases of Carcinoma of Palatine TonsilDocument5 pagesUnusual Cases of Carcinoma of Palatine TonsilPridho GaziansyahNo ratings yet
- 18 Extra-GuptaNDocument3 pages18 Extra-GuptaNGalih rarang gatiNo ratings yet
- A Rare Case of A Giant Tumor of Sebaceous Glands in The Posterior Chest WallDocument7 pagesA Rare Case of A Giant Tumor of Sebaceous Glands in The Posterior Chest WallAthenaeum Scientific PublishersNo ratings yet
- Follicular Dendritic Cell Sarcoma of The Tonssilar About A CaseDocument4 pagesFollicular Dendritic Cell Sarcoma of The Tonssilar About A CaseInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Cystic Mass of The Floor of The MouthDocument4 pagesCystic Mass of The Floor of The MouthMARIA KARINA HERNANDEZ PEREZNo ratings yet
- 下颌颊分叉囊肿 2例病例报告(英文) - 刘耀然.caj 2023 02 14 14 16 20 213Document3 pages下颌颊分叉囊肿 2例病例报告(英文) - 刘耀然.caj 2023 02 14 14 16 20 213朱嘉琪No ratings yet
- 1808 8694 Bjorl 86 s1 0s23Document3 pages1808 8694 Bjorl 86 s1 0s23VITOR PEREZNo ratings yet
- Odontogenic Myxoma A Rare Case ReportDocument3 pagesOdontogenic Myxoma A Rare Case Reportvictorubong404No ratings yet
- A Case of Maxillary Sinus CarcinomaDocument3 pagesA Case of Maxillary Sinus CarcinomajasmniaNo ratings yet
- F0605yDocument6 pagesF0605ySetiaty PandiaNo ratings yet
- Recurrent Abscesses of The Neck: Scrofuloderma: Bacterium Tuberculosis PCR Were Negative. A Culture Was NotDocument2 pagesRecurrent Abscesses of The Neck: Scrofuloderma: Bacterium Tuberculosis PCR Were Negative. A Culture Was NothadriantiNo ratings yet
- CEOTDocument8 pagesCEOTDrSahar PakzadNo ratings yet
- 2AMELOBLASTOMADocument5 pages2AMELOBLASTOMAROSVITA EUFEMIA SANCHEZ VALENZUELANo ratings yet
- Granular Cell Tumor of The Tongue A Case Report With Emphasis On Thediagnostic and Therapeutic Proceedings OCCRS 1000106Document3 pagesGranular Cell Tumor of The Tongue A Case Report With Emphasis On Thediagnostic and Therapeutic Proceedings OCCRS 1000106arypwNo ratings yet
- Metastastic Vulvar Squamous Cell Carcinoma Mimicking Genital HerpesDocument3 pagesMetastastic Vulvar Squamous Cell Carcinoma Mimicking Genital HerpesSteve EdwardNo ratings yet
- Ameloblastomatous Calcifying Odontogenic Cyst: A Case Report of A Rare Histologic VariantDocument4 pagesAmeloblastomatous Calcifying Odontogenic Cyst: A Case Report of A Rare Histologic VariantindhupriyakNo ratings yet
- Adenoid Cystic Carcinoma of The Oral CavityDocument5 pagesAdenoid Cystic Carcinoma of The Oral CavityshokoNo ratings yet
- Odontogenic Keratocyst Diagnosis and Management A Case ReportDocument5 pagesOdontogenic Keratocyst Diagnosis and Management A Case ReportInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Recurrence Odontogenic Keratocyst of The Mandible Treated by Surgical Hemimandibulectomy: Case ReportDocument10 pagesRecurrence Odontogenic Keratocyst of The Mandible Treated by Surgical Hemimandibulectomy: Case Reportarbi123No ratings yet
- Surgical Treatment of Dermatofibrosarcoma Protubera 2017 Journal of Dental SDocument2 pagesSurgical Treatment of Dermatofibrosarcoma Protubera 2017 Journal of Dental SAbdelwahed MoujaneNo ratings yet
- Tabrizi2012marsupialization As A Treatment Option For The Odontogenic KeratocystDocument3 pagesTabrizi2012marsupialization As A Treatment Option For The Odontogenic KeratocystquetmauhamNo ratings yet
- Locally Advanced Oral Kaposis Sarcoma in An Hiv Negative Woman Treated With Exclusive Radiotherapy: Case ReportDocument5 pagesLocally Advanced Oral Kaposis Sarcoma in An Hiv Negative Woman Treated With Exclusive Radiotherapy: Case ReportIJAR JOURNALNo ratings yet
- 1 Case ReportDocument3 pages1 Case ReportbarcimNo ratings yet
- Odontogenic Myxoma in A Child: Diagnostic and Treatment DilemmasDocument5 pagesOdontogenic Myxoma in A Child: Diagnostic and Treatment DilemmasthedocperuNo ratings yet
- Bauer 2007Document2 pagesBauer 2007gokberkuyariphoneNo ratings yet
- Rhinomaxillary Mucormycosis Clinical Signs and SymptomsDocument42 pagesRhinomaxillary Mucormycosis Clinical Signs and SymptomsAli FaridiNo ratings yet
- Implications of Endodontic Treatment In.4Document8 pagesImplications of Endodontic Treatment In.4Ali FaridiNo ratings yet
- Int Endodontic J - 2023 - DuncanDocument58 pagesInt Endodontic J - 2023 - DuncanAli FaridiNo ratings yet
- Endodontics: Developments inDocument17 pagesEndodontics: Developments inAli FaridiNo ratings yet
- Restorative Dentistry: Developments inDocument21 pagesRestorative Dentistry: Developments inAli Faridi100% (1)
- Ebook: Bioactive Dental Materials: The Future Is NowDocument7 pagesEbook: Bioactive Dental Materials: The Future Is NowAli FaridiNo ratings yet
- Reliability and Failure Modes of A Hybrid Ceramic Abutment PrototypeDocument5 pagesReliability and Failure Modes of A Hybrid Ceramic Abutment PrototypeAli FaridiNo ratings yet
- Comprehensive Evaluation of Knowledge and Perceptions Regarding Geriatric Dentistry Among Saudi Arabian Dental StudentsDocument11 pagesComprehensive Evaluation of Knowledge and Perceptions Regarding Geriatric Dentistry Among Saudi Arabian Dental StudentsAli FaridiNo ratings yet
- Effectiveness of Propolis in Maintaining Oral HealthDocument10 pagesEffectiveness of Propolis in Maintaining Oral HealthAli FaridiNo ratings yet
- Advanced Restorative Dentistry A Problem For The ElderlyDocument8 pagesAdvanced Restorative Dentistry A Problem For The ElderlyAli Faridi100% (1)
- ENdo FellowshipDocument2 pagesENdo FellowshipAli FaridiNo ratings yet
- Biomechanics of Removable of Partial DenturesDocument26 pagesBiomechanics of Removable of Partial DenturesAli Faridi67% (3)
- New Clasp Assembly For Distal Extension Removable Partial DenturesDocument17 pagesNew Clasp Assembly For Distal Extension Removable Partial DenturesAli FaridiNo ratings yet
- Risk Factors Contributing To Symptomatic Plate Removal in Orthognathic Surgery PatientsDocument4 pagesRisk Factors Contributing To Symptomatic Plate Removal in Orthognathic Surgery PatientsAli FaridiNo ratings yet
- Six Step Approach To Curriculum DevelopmentDocument86 pagesSix Step Approach To Curriculum DevelopmentAli FaridiNo ratings yet
- All Ceramic InlaysDocument14 pagesAll Ceramic InlaysAli FaridiNo ratings yet
- Selectins: Dr. Muhammad Ali Faridi Dr. Arathy JosephDocument17 pagesSelectins: Dr. Muhammad Ali Faridi Dr. Arathy JosephAli FaridiNo ratings yet
- Flexural Strength Comparison of GCP and GICDocument22 pagesFlexural Strength Comparison of GCP and GICAli FaridiNo ratings yet
- Prosthodontics OspeDocument30 pagesProsthodontics OspeAli Faridi86% (7)
- Phaco Vs Ecce Tatjana PDFDocument4 pagesPhaco Vs Ecce Tatjana PDFfariza9rahma9safiraNo ratings yet
- Factors Affecting Time of USCODocument10 pagesFactors Affecting Time of USCOMUBENGANo ratings yet
- Westmead Obstetric AnaestheDocument85 pagesWestmead Obstetric Anaesthesum75No ratings yet
- Git Bleeding - Upper & Lower.Document36 pagesGit Bleeding - Upper & Lower.Muwanga faizoNo ratings yet
- Esthetic Restoration of The ITI Implant: Richard P. Kinsel, DDSDocument59 pagesEsthetic Restoration of The ITI Implant: Richard P. Kinsel, DDSLouis HutahaeanNo ratings yet
- 1.enbloc Resection of Mandible As Treatment of Ameloblastoma - A Review With Case ReportDocument2 pages1.enbloc Resection of Mandible As Treatment of Ameloblastoma - A Review With Case ReportcoklatstrawberryNo ratings yet
- Medical Triads, Tetrads, and PentadsDocument10 pagesMedical Triads, Tetrads, and PentadsAyessa BandalNo ratings yet
- Prolaps UteriDocument7 pagesProlaps Uteridr.raziNo ratings yet
- Scheuermanns DiseaseDocument26 pagesScheuermanns DiseaseMihnea VulpeNo ratings yet
- Clinical Applications of The Venous Excess Ultrasound (Vexus) Score: Conceptual Review and Case SeriesDocument10 pagesClinical Applications of The Venous Excess Ultrasound (Vexus) Score: Conceptual Review and Case SeriesPablo Sanchez MartinezNo ratings yet
- Pancreas and SpleenDocument106 pagesPancreas and SpleenJorge De Vera100% (1)
- RUBRIC Male Catheterization 1Document2 pagesRUBRIC Male Catheterization 1MELODY GATDULANo ratings yet
- Herniated Nucleus Pulposus: By: Baron Ablang Giselle Cruz Denise Dela Cruz Ronnel MactalDocument34 pagesHerniated Nucleus Pulposus: By: Baron Ablang Giselle Cruz Denise Dela Cruz Ronnel MactalDenise De la Cruz100% (1)
- MassDocument8 pagesMassYara YousefNo ratings yet
- Evaluasi Usg Dan Tatalaksana Pada Varises Vena Tungkai - DR RamziDocument31 pagesEvaluasi Usg Dan Tatalaksana Pada Varises Vena Tungkai - DR RamziMohamad ZulfikarNo ratings yet
- NCR 2018 Quirino Memorial Medical CenterDocument81 pagesNCR 2018 Quirino Memorial Medical CenterChristian LaranoNo ratings yet
- Medical Emergency in Pediatric PatientsDocument36 pagesMedical Emergency in Pediatric Patientsdrdeepikajoash16No ratings yet
- Critical Evaluation of Suture Materials and Suturing Techniques in Implant Dentistry PDFDocument10 pagesCritical Evaluation of Suture Materials and Suturing Techniques in Implant Dentistry PDFria mayantiNo ratings yet
- Types of AnesthesiaDocument27 pagesTypes of Anesthesiasncmanguiat.2202276.chasnNo ratings yet
- 14 - EKG-kegawatanDocument63 pages14 - EKG-kegawatanIka HayaturrohmahNo ratings yet
- Treatment of Neglected Syndesmotic InjuriesDocument16 pagesTreatment of Neglected Syndesmotic InjuriesMohamed GoudaNo ratings yet
- Barlows Syndrome PPT AbhishekDocument10 pagesBarlows Syndrome PPT AbhishekAbhishek SagarNo ratings yet
- Explore LapDocument5 pagesExplore LapAngie MandeoyaNo ratings yet
- 002-Trauma & BurnsDocument36 pages002-Trauma & BurnsAhmed Zaghw100% (2)
- Finished Head Injury Ppt-1Document76 pagesFinished Head Injury Ppt-1Chalie MequanentNo ratings yet
- Orca Share Media1466317404971Document122 pagesOrca Share Media1466317404971ShidevNo ratings yet
- Orthognathic Surgery Oral SurgeryDocument38 pagesOrthognathic Surgery Oral SurgeryMariam Tariq0% (1)
- Surgery OSCE QuestionsDocument27 pagesSurgery OSCE QuestionsSadia YousafNo ratings yet
- Ineffective Airway ClearanceDocument10 pagesIneffective Airway ClearanceHannah VueltaNo ratings yet
- The Stomach Can Be Divided Into Three Anatomic (A) and Two Functional Regions (B)Document31 pagesThe Stomach Can Be Divided Into Three Anatomic (A) and Two Functional Regions (B)emeredinNo ratings yet