Professional Documents
Culture Documents
The Atom
Uploaded by
JenniferOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
The Atom
Uploaded by
JenniferCopyright:
Available Formats
SNC 1DI Date: ______________________
The Atom
What is an atom?
• an atom is the _________________ part of an element that has all of the element’s_____________________
• for example, atoms of _______________________ are not the same as atoms of _______________.
This is why a piece of copper has different _______________________________ than a piece of iron
• atoms are made of smaller particles called __________________________________ particles;
protons, electrons, and neutrons
Properties of Subatomic Particles
Name Symbol Relative Mass Electric Charge Location
The Importance of Protons
• protons are especially important because the number of protons in an atom determines what
________________________________it is
• any atom with _______ proton is always ____________________________
• any atom with _______ protons is always ____________________________
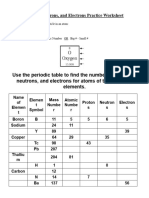
Determining the Number of Subatomic Particles in Each Atom
Use your periodic table to complete the table below.
Element Atomic Atomic Number of Number of Number of
Name Number Mass Protons Electrons Neutrons
Aluminum
Argon
40
Carbon
18
19
12
Neon
31
Sodium
You might also like
- Worksheet: Atoms, Isotopes, and Ions AtomsDocument2 pagesWorksheet: Atoms, Isotopes, and Ions AtomsLeo Torres GarcíaNo ratings yet
- Oxy-Acetylene Welding and Cutting: Electric, Forge and Thermit Welding together with related methods and materials used in metal working and the oxygen process for removal of carbonFrom EverandOxy-Acetylene Welding and Cutting: Electric, Forge and Thermit Welding together with related methods and materials used in metal working and the oxygen process for removal of carbonRating: 4 out of 5 stars4/5 (1)
- Oxy-Acetylene Welding and Cutting Electric, Forge and Thermit Welding together with related methods and materials used in metal working and the oxygen process for removal of carbonFrom EverandOxy-Acetylene Welding and Cutting Electric, Forge and Thermit Welding together with related methods and materials used in metal working and the oxygen process for removal of carbonNo ratings yet
- Lewis-Dot-Diagram-Worksheet - With AnswersDocument4 pagesLewis-Dot-Diagram-Worksheet - With AnswersRazan Ghalayini100% (1)
- Atomic Structure WorksheetDocument2 pagesAtomic Structure WorksheetMaan Ahmed Ba ShuraihNo ratings yet
- Atomic Structure: Atom Properties and Isotopes: Symbol With AN & MNDocument1 pageAtomic Structure: Atom Properties and Isotopes: Symbol With AN & MNFred EspejoNo ratings yet
- CHM ART Activity 4 Atoms and Isotopes PDFDocument4 pagesCHM ART Activity 4 Atoms and Isotopes PDFMark Vincent DoriaNo ratings yet
- Families of Elements ReviewDocument4 pagesFamilies of Elements ReviewdavenNo ratings yet
- 14-15 Basic Atomic Structure WorksheetDocument3 pages14-15 Basic Atomic Structure WorksheetAira EvangelistaNo ratings yet
- 2021 08 25 Atomic Notation Practice WorksheetDocument3 pages2021 08 25 Atomic Notation Practice WorksheetTimothy Urtz (Chicago)No ratings yet
- Periodic Table Bohr PracticeDocument2 pagesPeriodic Table Bohr PracticeForla MiNo ratings yet
- Atom QuizDocument1 pageAtom QuizRose Ann BernardoNo ratings yet
- Complete The FollowingDocument4 pagesComplete The FollowingdjdjdjdddjdjdNo ratings yet
- B 32 - 00 - QjmylvjfraDocument11 pagesB 32 - 00 - QjmylvjfraAndresNo ratings yet
- Counting Subatomic Particles and Calculating Average Atomic Mass AssignmentDocument3 pagesCounting Subatomic Particles and Calculating Average Atomic Mass AssignmentDamien WhitakerNo ratings yet
- Annotated-Atomic Structure Bohr Models-1Document2 pagesAnnotated-Atomic Structure Bohr Models-1Ivania Joselina Lobo MontoyaNo ratings yet
- CHM150 (Practice) (Atomic and Mass Number)Document1 pageCHM150 (Practice) (Atomic and Mass Number)sandraNo ratings yet
- Yoga Agung Saputra 10/1/2016: Designed by Checked by Approved by Date DateDocument2 pagesYoga Agung Saputra 10/1/2016: Designed by Checked by Approved by Date DateAbu SufyanNo ratings yet
- Chapter 3 - SeatworkDocument2 pagesChapter 3 - SeatworkVincent DayangcoNo ratings yet
- Unit 03 HW PacketDocument21 pagesUnit 03 HW Packetanabel mañoNo ratings yet
- Science8 Q3 Week6Document20 pagesScience8 Q3 Week6Kathrina De SenaNo ratings yet
- Periodic Table Unit PacketDocument5 pagesPeriodic Table Unit PacketFlvcko SlimNo ratings yet
- Subatomic Particles - FILLDocument2 pagesSubatomic Particles - FILLALMERA SHELLA CABOGONo ratings yet
- Group Activity1Document1 pageGroup Activity1ansalerochNo ratings yet
- HW 7 Answer KeyDocument2 pagesHW 7 Answer Keyangelyn martinezNo ratings yet
- Atom Structure WSDocument6 pagesAtom Structure WSRoxana RuizNo ratings yet
- Chemistry Lesson PacketDocument12 pagesChemistry Lesson PacketLauren MorrissNo ratings yet
- Worksheet 4Document2 pagesWorksheet 4Joan RosanaNo ratings yet
- Pyrometallurgical Refining of Copper in An Anode Furnace: January 2005Document13 pagesPyrometallurgical Refining of Copper in An Anode Furnace: January 2005maxi roaNo ratings yet
- Joshua Perez-Luna - Isotopes Review Sheet PDFDocument2 pagesJoshua Perez-Luna - Isotopes Review Sheet PDFJoshua Perez-LunaNo ratings yet
- Kami Export - Jaeden Reames - Periodic Table Unit Packet PracticeDocument5 pagesKami Export - Jaeden Reames - Periodic Table Unit Packet PracticeJaeden ReamesNo ratings yet
- ATOMIC STRUCTURE-moduleDocument6 pagesATOMIC STRUCTURE-modulejudith cue100% (1)
- MN SoADocument22 pagesMN SoAjoe bloggNo ratings yet
- 01 - Atomic Theory Notes 2012 - KeyDocument2 pages01 - Atomic Theory Notes 2012 - Keyapi-292000448No ratings yet
- Perioidic Table Packet - 1 1 2Document5 pagesPerioidic Table Packet - 1 1 2Deema AljaririNo ratings yet
- Kelapa GadingDocument2 pagesKelapa GadingUtari Ika CahyaniNo ratings yet
- Essential Trends in Physical and Chemical properties-WorkSheetDocument10 pagesEssential Trends in Physical and Chemical properties-WorkSheetFELIX ORATINo ratings yet
- Nuclear Chemistry Problems-1Document4 pagesNuclear Chemistry Problems-1Marques CatheyNo ratings yet
- Perioidic Table Packet - 1 1 2Document4 pagesPerioidic Table Packet - 1 1 2jelly marie floresNo ratings yet
- Power Scaling of Kw-Diode Lasers Optimized For Material Processing ApplicationsDocument9 pagesPower Scaling of Kw-Diode Lasers Optimized For Material Processing ApplicationsDu RoyNo ratings yet
- Chapter 02 ISM Chang 14eDocument7 pagesChapter 02 ISM Chang 14elsytb2000No ratings yet
- NS - Atom Elements Reactions and MixturesDocument11 pagesNS - Atom Elements Reactions and MixturesChantal JansenNo ratings yet
- Build An AtomDocument2 pagesBuild An Atomapi-553259322No ratings yet
- Atomic: StructureDocument1 pageAtomic: StructureJovariya RaziqNo ratings yet
- Atomic Structure & The Periodic Table 1 QPDocument8 pagesAtomic Structure & The Periodic Table 1 QPAisha Jakhro100% (1)
- Solution Manual For Chemistry 11th Edition by Chang ISBN 007766695X 9780077666958Document36 pagesSolution Manual For Chemistry 11th Edition by Chang ISBN 007766695X 9780077666958henryarmstrongypajbizoqe100% (23)
- Activity 2 PDFDocument4 pagesActivity 2 PDFjenervas0102No ratings yet
- Atomic Radius Graphing ActivityDocument2 pagesAtomic Radius Graphing ActivitytweetyaarushiNo ratings yet
- Basic 1st 01 02Document8 pagesBasic 1st 01 02Taseem Ali KhanNo ratings yet
- Atomic MassDocument1 pageAtomic MassDeepti JainNo ratings yet
- Science8 QuizDocument2 pagesScience8 QuizMichaelAbdonDomingoFavoNo ratings yet
- Bat-Dwg-Dd-Me-00-Ep-6002 - R0 - Load Schedule Diagram of PP - LP - Building 32Document1 pageBat-Dwg-Dd-Me-00-Ep-6002 - R0 - Load Schedule Diagram of PP - LP - Building 32CosphiiiNo ratings yet
- Solution Manual For Chemistry 11Th Edition by Chang Isbn 007766695X 9780077666958 Full Chapter PDFDocument36 pagesSolution Manual For Chemistry 11Th Edition by Chang Isbn 007766695X 9780077666958 Full Chapter PDFtiffany.kunst387100% (10)
- Atomic Structure WorksheetsDocument3 pagesAtomic Structure WorksheetsJohnaire RowellNo ratings yet
- What Is An Atom? What Are The Three Parts That Make Up An Atom? 1. 2. 3. What Does An Atom Look Like?Document6 pagesWhat Is An Atom? What Are The Three Parts That Make Up An Atom? 1. 2. 3. What Does An Atom Look Like?VarshLokNo ratings yet
- 09 Petrucci10e SSM PDFDocument17 pages09 Petrucci10e SSM PDFkennethleo69No ratings yet
- W.atoms and IonsDocument2 pagesW.atoms and IonsMahmoud AladdasiNo ratings yet
- Chapter 3 (Notes and Activities)Document10 pagesChapter 3 (Notes and Activities)Ahmed MutwakilNo ratings yet
- Unusual Structures and Physical Properties in Organometallic ChemistryFrom EverandUnusual Structures and Physical Properties in Organometallic ChemistryNo ratings yet