Professional Documents
Culture Documents
Biochem Laboratory Reviewer
Uploaded by
2023700920 ratings0% found this document useful (0 votes)
22 views6 pagesOriginal Title
BIOCHEM LABORATORY REVIEWER
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
22 views6 pagesBiochem Laboratory Reviewer
Uploaded by
202370092Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 6
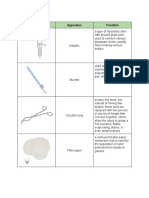
BIOCHEM LABORATORY o Stirring Rod: used for stirring
liquids or mixing substances in
laboratory experiments.
Laboratory Equipment
o Test Tube: hold small amounts of o Volumetric Flask: designed to hold
liquids or substances for various a specific volume of liquid
laboratory experiments and tests. at a precise temperature.
o Distilling Flask: used for distillation
o Test Tube Rack: a rack or holder
processes to separate and collect
designed to hold multiple test tubes
components of a mixture based on
upright.
their boiling points.
o Test Tube Holder: used to hold
o Wire Gauze: often placed on top of
individual test tubes securely,
a Bunsen burner or other heat source
especially when they are hot.
to support glassware during heating.
o Test Tube Brush: used for cleaning
o Spatula: used for transferring small
the inside of test tubes.
amounts of solid chemicals or
substances.
o Beaker: used for measuring, mixing,
and heating liquids and solutions.
o Watch Glass: used as a cover
or for evaporating small amounts of
liquid.
o Erlenmeyer Flask: used for
titration, mixing, and heating liquids.
o Crucible with Cover: used for
heating substances to high
o Graduated Cylinder: used to
temperatures.
measure the volume of liquids
accurately.
o Pipette: used for precise
measurement and transfer of
o Weighing Scale: used to measure the
small volumes of liquid.
mass or weight of objects or
substances with high precision.
o Crucible Tongs: designed to handle o Mortar & Pestle: used for grinding
crucibles safely, especially when and mixing substances, especially
they are hot. solids, to create a fine powder or
homogenous mixture.
Questions:
o Hot Plate: used for heating
substances in laboratory 1. Which glasswares are used in measuring
experiments, often with volume?
adjustable temperature settings.
2. Which of these glasswares measure the
most accurate volume?
o Iron Stand: used to hold 3. Which apparatuses are used for heating a
various laboratory equipment, setup?
such as clamps and supports. 4. Give some safety measures when working
in a laboratory, especially when using
glasswares.
o Iron Ring: an iron stand to
support laboratory glassware,
such as a funnel or flask. Diffusion: the process by which particles or
molecules move from an area of higher
concentration to an area of lower
o Burette: used for precise titration concentration.
of liquids.
o It occurs in various states of matter,
including gases, liquids, and solids.
o Evaporating Dish: used for the Diffusion happens due to the random
evaporation of liquids to concentrate motion of particles, driven by their
solutions or recover solids. kinetic energy.
Osmosis: is a specific type of diffusion that
involves the movement of water molecules
o Iron Clamp: used to secure across a selectively permeable membrane
laboratory glassware, such as a from an area of lower solute concentration to
burette or flask, to an iron stand. an area of higher solute concentration.
o In osmosis, water molecules move
through the membrane to equalize
o Micropipette: used for dispensing
the concentration of solute particles
very small volumes of liquid, often
on both sides of the membrane. It is a
in microliters.
fundamental process in biology and
plays a crucial role in various
biological and physiological
o Triple Beam Balance: for
processes.
measuring mass with high accuracy.
Dialysis: Dialysis is a medical and solutions of different salt concentrations. It
laboratory procedure used to remove waste should illustrate how water moves into or
products and excess fluids from the blood out of the potato cells depending on the
when the kidneys are unable to perform this surrounding salt concentration, leading to
function adequately. changes in the size and texture of the potato
slices.
Title: Osmosis in Potato Cells
Objective: To observe and investigate the
process of osmosis in plant cells by placing
potato slices in solutions of varying salt Title: Crystallization of Naphthalene Balls
concentrations.
Incubation: Allow the potato slices to soak
in the solutions for a set period, such as 30 Objective: To demonstrate the process of
minutes to 1 hour. Ensure that the conditions crystallization by dissolving naphthalene
(temperature, light) are the same for all balls in a solvent and then allowing the
containers. naphthalene to re-crystallize as the solution
cools.
Observation: After the incubation period,
remove the potato slices from the solutions Procedure:
and blot them gently with paper towels to 1. Safety Precautions
remove excess solution.
- Put on safety goggles and laboratory gloves
Observe and compare the appearance of the to protect your eyes and skin.
potato slices in each container. Note any
2. Preparation:
changes in size, texture, or color.
- Weigh a small amount (about 10-15 grams)
Analysis and Discussion: Compare the
of naphthalene balls using a balance and record
initial and final masses of the potato slices in the exact mass.
each solution.
3. Dissolution:
o Discuss the changes observed in the
potato slices and explain them in - Place the naphthalene balls in a clean, dry
glass beaker.
terms of osmosis.
o Which potato slices gained mass, - Heat a small amount of distilled water in
which lost mass, and which remained another beaker until it is just below boiling
relatively unchanged? temperature (approximately 80-90°C).
o Explain why these changes occurred - Carefully pour the hot water over the
based on the movement of water naphthalene balls in the beaker. Use just enough
molecules in response to the salt water to completely cover the naphthalene balls.
concentration. Stir the mixture gently with a glass stirring rod
until the naphthalene balls dissolve completely.
This experiment demonstrates the effects of
osmosis on plant cells by placing them in 4. Cooling and Crystallization:
- Allow the naphthalene solution to cool in a hot solvent and then allowing it to re-
gradually to room temperature. You can speed crystallize as the solution cools. It also
up this process by placing the beaker in an ice allows for discussions on the concepts of
bath. solubility, supersaturation, and the
- As the solution cools, observe the formation formation of pure crystals from a solution.
of naphthalene crystals. They should start to
appear as the solution cools down.
5. Filtration:
- Once the solution has cooled and
crystallization is evident, set up a filtration
apparatus with a funnel and filter paper over
another clean beaker.
- Carefully pour the solution through the filter
paper to separate the naphthalene crystals from Volume Transfer (pipette vs test tube)
the liquid.
Volume transfer in a laboratory setting can
6. Collection of Crystals: be accomplished using various tools,
- Remove the filter paper with the naphthalene including pipettes and test tubes. Here, we'll
crystals and place it on a watch glass or Petri discuss the differences between these two
dish to allow the crystals to air-dry completely. methods for volume transfer:
7. Mass Measurement:
- After the crystals have dried, weigh them 1. Pipette
using a balance and record the mass.
o Precision and Accuracy: Pipettes are
Analysis and Discussion: highly precise and accurate
1. Calculate the percent recovery of instruments for transferring known
naphthalene by comparing the mass of the volumes of liquids. They are
recovered crystals to the initial mass of calibrated to deliver a specific
naphthalene balls used. volume, such as 10 mL or 100 µL,
with high accuracy.
2. Discuss the process of crystallization and o Volume Range: Pipettes come in a
how it occurs in this experiment. wide range of sizes to accommodate
3. Explain why hot water is used to dissolve different volume requirements, from
the naphthalene balls and why the solution is microliters (µL) to milliliters (mL).
allowed to cool slowly. o Controlled Dispensing: Pipettes
allow for precise control over the
4. What factors could affect the yield of volume being transferred. This is
crystals in this experiment? especially important when handling
small volumes or when high
accuracy is required.
This experiment demonstrates the principles o Disposable Tips: Many pipettes use
of crystallization by dissolving naphthalene disposable tips, which reduce the risk
of cross-contamination between used in chemistry, biology, and
samples. microbiology laboratories.
o Common Types: Common types of
pipettes include micropipettes (for
microliter volumes), serological In summary, pipettes are specialized
pipettes (for milliliter volumes), and instruments designed for precise and
volumetric pipettes (for very accurate volume transfer, making them
accurate measurements). suitable for tasks that require exact
o Applications: Pipettes are often used measurements. Test tubes, on the other hand,
in analytical chemistry, molecular are versatile containers used for a wide
biology, and medical laboratories for range of laboratory purposes but are
tasks like measuring reagents, generally less precise for volume transfer.
transferring samples, and making The choice between pipettes and test tubes
dilutions. depends on the specific requirements of the
experiment and the desired level of accuracy
and precision.
2. Test Tube
o Precision and Accuracy: Test tubes
are not as precise or accurate as
pipettes when it comes to volume
transfer. They are typically used for
rough volume estimates rather than
precise measurements.
o Volume Range: Test tubes come in
various sizes, but their volume
measurements are typically not as
exact as those of pipettes.
o Controlled Dispensing: Test tubes do
not offer the same level of control
over volume dispensing as pipettes.
Pouring liquids into a test tube can
result in spillage and less accurate
measurements.
o Reusable: Test tubes are typically
reusable, which can be cost-
effective. However, this can also lead
to the risk of cross-contamination if
not cleaned thoroughly.
o Applications: Test tubes are
commonly used for mixing, heating,
and storing small to moderate
volumes of liquids. They are often
You might also like
- Chemistry Lab Mysteries, Fun Laboratory Tools! Chemistry for Kids - Children's Analytic Chemistry BooksFrom EverandChemistry Lab Mysteries, Fun Laboratory Tools! Chemistry for Kids - Children's Analytic Chemistry BooksRating: 5 out of 5 stars5/5 (1)
- Practical Lecture 2 Consumables and Instruments in Biochemistry LaboratoryDocument51 pagesPractical Lecture 2 Consumables and Instruments in Biochemistry LaboratoryMRM7MDNo ratings yet
- Stress-Free Science: A Visual Guide to Acing Science in Grades 4-8From EverandStress-Free Science: A Visual Guide to Acing Science in Grades 4-8No ratings yet
- It Is Used For Mixing, Stirring, and Heating ChemicalsDocument7 pagesIt Is Used For Mixing, Stirring, and Heating ChemicalsDessa GuditoNo ratings yet
- Dry Lab Inorganic ChemistryDocument7 pagesDry Lab Inorganic ChemistryAbegeil RadimaNo ratings yet
- Laboratory Equipment and FunctionsDocument4 pagesLaboratory Equipment and FunctionsDiane DimaalaNo ratings yet
- Chem181: Chemistry For Engineers - Laboratory First Semester, AY 2020-2021 (Cluster 1) Activity No. 1 Common Laboratory ApparatusDocument5 pagesChem181: Chemistry For Engineers - Laboratory First Semester, AY 2020-2021 (Cluster 1) Activity No. 1 Common Laboratory ApparatusMaxine de la TorreNo ratings yet
- Lab ApparatusDocument10 pagesLab ApparatuslizNo ratings yet
- Commonly Encountered Equipment in The Chemistry LaboratoryDocument11 pagesCommonly Encountered Equipment in The Chemistry LaboratoryAudrey MendozaNo ratings yet
- Phar 205Document156 pagesPhar 205billhaddNo ratings yet
- Lab EquipmentsDocument10 pagesLab Equipmentschouroukbelkacemi236No ratings yet
- Name Picture Uses Utility Clamp: Used To Secure Glassware To A Ring StandDocument8 pagesName Picture Uses Utility Clamp: Used To Secure Glassware To A Ring StandRyan Jules DaisNo ratings yet
- Laboratory Equipment and FunctionsDocument5 pagesLaboratory Equipment and FunctionsIraqiNo ratings yet
- Matter and Kinetic Theory of Matter Power PointDocument64 pagesMatter and Kinetic Theory of Matter Power PointAkaNayep ApNo ratings yet
- Microbiology Laboratory Equipment and Functions: Ring StandDocument6 pagesMicrobiology Laboratory Equipment and Functions: Ring StandHector CandidoNo ratings yet
- Commonly Encountered Equipment in The Chemistry LaboratoryDocument7 pagesCommonly Encountered Equipment in The Chemistry LaboratoryKIRBY ANN GALABINNo ratings yet
- Basic Laboratory Glassware and EquipmentDocument5 pagesBasic Laboratory Glassware and EquipmentEj RafaelNo ratings yet
- Diccionario de Laboratorio 1Document5 pagesDiccionario de Laboratorio 1dadadadNo ratings yet
- Chemistry Laboratory: Group 1Document51 pagesChemistry Laboratory: Group 1Rachel Ann De LeonNo ratings yet
- Activity 1Document4 pagesActivity 1Hannah VisitacionNo ratings yet
- Mon Lab ApparatusDocument11 pagesMon Lab ApparatusJustin MenorasNo ratings yet
- Lab Equipment BSCrim Organic - AutosavedDocument130 pagesLab Equipment BSCrim Organic - Autosavedangelo aquinoNo ratings yet
- MYP - 1-Lab - Equipment - PPTDocument53 pagesMYP - 1-Lab - Equipment - PPTvandana giriNo ratings yet
- CHEM Midterm NotesDocument7 pagesCHEM Midterm NotesKharylle Ann IglesiasNo ratings yet
- Laboratory ApparatusDocument10 pagesLaboratory ApparatusSEAN GAVIN DELOS REYESNo ratings yet
- Laboratory Equipment and FunctionsDocument4 pagesLaboratory Equipment and FunctionsAngeline Panaligan Ansela100% (2)
- Commonly Encountered Equipment in The Chemistry LaboratoryDocument9 pagesCommonly Encountered Equipment in The Chemistry Laboratorysadiqah mushtaqNo ratings yet
- InorglabequipmentsDocument7 pagesInorglabequipmentsLOUISE RICA LAGAHITNo ratings yet
- Experiment 1 - Laboratory Apparatus 1Document6 pagesExperiment 1 - Laboratory Apparatus 1Raymart DomingoNo ratings yet
- CHEM2212 - ESTANOCO - SAMUEL.M. - Activity 1 LABORATORY EQUIPMENTDocument6 pagesCHEM2212 - ESTANOCO - SAMUEL.M. - Activity 1 LABORATORY EQUIPMENTSam EstanocoNo ratings yet
- Bunsen Burner A Watch GlassDocument4 pagesBunsen Burner A Watch GlassAna Soriano DocoyNo ratings yet
- A Container For Culturing Cells or MicrobesDocument4 pagesA Container For Culturing Cells or Microbesindah LatifaNo ratings yet
- Assignment: Inorganic and Organic Chemistry CHM101Document3 pagesAssignment: Inorganic and Organic Chemistry CHM101Cj DelfinNo ratings yet
- Chemistry For Engineers (NS 111) : SafetyDocument11 pagesChemistry For Engineers (NS 111) : SafetyMark Angelo Belza SotoNo ratings yet
- Laboratory EquipmentDocument3 pagesLaboratory Equipmenttrishamaebosque68No ratings yet
- Lab ApparatusDocument4 pagesLab ApparatusArafhel AnciroNo ratings yet
- Ed. LABORATORY-MANUAL-CHEM-1108Document74 pagesEd. LABORATORY-MANUAL-CHEM-1108Joevelyn ValdezNo ratings yet
- Dr. Zarina Arif: Assistant Professor D/O Biochemistry J.N.M.C., A.M.UDocument67 pagesDr. Zarina Arif: Assistant Professor D/O Biochemistry J.N.M.C., A.M.URuchieNo ratings yet
- Balance Bunsen BurnerDocument5 pagesBalance Bunsen BurnerCharlie NipaNo ratings yet
- Activity 2 Glasswares 2Document5 pagesActivity 2 Glasswares 2EDCEL PEDRONo ratings yet
- Name Description PictureDocument4 pagesName Description PictureHazel AjeroNo ratings yet
- SSC Basic Laboratory Tools PDFDocument52 pagesSSC Basic Laboratory Tools PDFKim Dorothy DayagNo ratings yet
- Barecuatro - BSN1-11L - Preliminary ActivitiesDocument19 pagesBarecuatro - BSN1-11L - Preliminary ActivitiesAngelica Claire BarecuatroNo ratings yet
- AnferneeDocument5 pagesAnferneeIrene Soriano BayubayNo ratings yet
- Laboratory ApparatusDocument5 pagesLaboratory ApparatusArantxa Hilario100% (1)
- Appendices: Appendix A: List and Uses of ApparatusDocument8 pagesAppendices: Appendix A: List and Uses of ApparatusFranzAlpha May Mandane BautistaNo ratings yet
- Name Picture Function: Jan Chrispian Mirasol Riza Mae Naria 1BSPH2Document9 pagesName Picture Function: Jan Chrispian Mirasol Riza Mae Naria 1BSPH2Jan Chrispian MirasolNo ratings yet
- Exercise 2 Familiarization of Laboratory ApparatusDocument4 pagesExercise 2 Familiarization of Laboratory ApparatusESTELLE RHINE HINDAP FRANCISCO100% (1)
- Lab EquipmentDocument8 pagesLab EquipmentMathew YoyakkyNo ratings yet
- BioChemistry Manual DelosReysDocument21 pagesBioChemistry Manual DelosReysVALERIE JOY CATUDIONo ratings yet
- Adobe Scan Nov 25, 2022Document8 pagesAdobe Scan Nov 25, 2022Mayank GoyalNo ratings yet
- Name Picture Function: Jan Chrispian Mirasol Riza Mae Naria 1BSPH2Document9 pagesName Picture Function: Jan Chrispian Mirasol Riza Mae Naria 1BSPH2Jan Chrispian MirasolNo ratings yet
- Lab - Apparatus FinalDocument119 pagesLab - Apparatus FinalChristian James MarianoNo ratings yet
- Laboratory Glassware and Apparatus LSMDocument4 pagesLaboratory Glassware and Apparatus LSMShiina Mashiro100% (1)
- Laboratory Equipment and Their FunctionsDocument42 pagesLaboratory Equipment and Their FunctionsAtika RachmasariNo ratings yet
- Group 3Document11 pagesGroup 3Kyle's ChannelNo ratings yet
- Igoy Ukulan ChemistryDocument8 pagesIgoy Ukulan ChemistryGenesis Ducusin AguilarNo ratings yet
- Lab Apparatus and The Do and Donts (Torrecampo, Joevic)Document2 pagesLab Apparatus and The Do and Donts (Torrecampo, Joevic)joevic torrecampoNo ratings yet
- Genchem La 1 SantanDocument10 pagesGenchem La 1 SantanDece Andrea SantanNo ratings yet
- 4Ws WTWW GOD IS FOR US Are You For God - SEPT 18 22 MAINDocument2 pages4Ws WTWW GOD IS FOR US Are You For God - SEPT 18 22 MAIN202370092No ratings yet
- A ManDocument1 pageA Man202370092No ratings yet
- HISTORIOGRAPHYDocument15 pagesHISTORIOGRAPHY202370092No ratings yet
- HISTORIOGRAPHYDocument15 pagesHISTORIOGRAPHY202370092No ratings yet
- Gas Injection Technology.Document49 pagesGas Injection Technology.Irfan AliNo ratings yet
- Benedict's Test For Non-Reducing SugarsDocument2 pagesBenedict's Test For Non-Reducing SugarsSamer Ehab75% (4)
- Steel P235TR2Document2 pagesSteel P235TR2Moulham ShahinNo ratings yet
- The Influence of UV Light On Photodegradation of Acetylsalicylic AcidDocument15 pagesThe Influence of UV Light On Photodegradation of Acetylsalicylic AcidNhím BiểnNo ratings yet
- To Determine Particle Size Distribution of Soil by SievingDocument7 pagesTo Determine Particle Size Distribution of Soil by SievingAhmad AbubakarNo ratings yet
- Chemistry Syllabus Karachi UniversityDocument102 pagesChemistry Syllabus Karachi UniversityAsna Masood0% (1)
- NoteDocument3 pagesNotemtam.ctrlNo ratings yet
- New Microsoft Office Word DocumentDocument9 pagesNew Microsoft Office Word DocumentSanthiya SanjeeviNo ratings yet
- Estimation of Iodine in Iodized Common SaltDocument10 pagesEstimation of Iodine in Iodized Common SaltRajesh ChoudharyNo ratings yet
- Presentation 2Document18 pagesPresentation 2rayonneeemdyNo ratings yet
- Pharmacognostic and Phytochemical Evaluation of Chrysophyllum Cainito Linn. LeavesDocument6 pagesPharmacognostic and Phytochemical Evaluation of Chrysophyllum Cainito Linn. LeavesWildan SuhadiNo ratings yet
- Makin (2019) PDSNDocument11 pagesMakin (2019) PDSNFabricio CarrilloNo ratings yet
- UNIT II Acid Base TitrationDocument48 pagesUNIT II Acid Base TitrationDr Priti JainNo ratings yet
- Chemical Test Instruction (27 Feburary 2017)Document9 pagesChemical Test Instruction (27 Feburary 2017)AmirulNo ratings yet
- Guided by Dr. R.Gandhimathi Done By: Billa Manasa Reddy Kavya Sharma Mainowshree Boro Shamma Anna JacobDocument13 pagesGuided by Dr. R.Gandhimathi Done By: Billa Manasa Reddy Kavya Sharma Mainowshree Boro Shamma Anna JacobManasa Reddy BillaNo ratings yet
- BoQ - Insulation Works (Group 1)Document5 pagesBoQ - Insulation Works (Group 1)Bhavanishankar ShettyNo ratings yet
- Ionic EquilibriumDocument100 pagesIonic EquilibriumShohom DeNo ratings yet
- MR - Bhushan Kharbadkar Project ReportDocument10 pagesMR - Bhushan Kharbadkar Project ReportPratik BhelondeNo ratings yet
- 10-Lab-10Spectrophotometric Determination of PhosphatDocument4 pages10-Lab-10Spectrophotometric Determination of PhosphatHoang Huong Tra33% (3)
- Hydrogen Bonding: Where To Find Hydrogen Bonds?Document28 pagesHydrogen Bonding: Where To Find Hydrogen Bonds?Rahul DhimanNo ratings yet
- Balancing Redox Reactions: Examples: MN O I I NDocument4 pagesBalancing Redox Reactions: Examples: MN O I I NSHENIVEL BANTENo ratings yet
- Rubber Process Oil - Residual Aromatic Extract (Exdo-4) DescriptionDocument1 pageRubber Process Oil - Residual Aromatic Extract (Exdo-4) DescriptionLeonie SaputriNo ratings yet
- A Guide To SS13 ChemistryDocument1 pageA Guide To SS13 Chemistrywhat about the wookiesNo ratings yet
- Aee Mechanical Gs 09-05-2023Document72 pagesAee Mechanical Gs 09-05-2023RaghuNo ratings yet
- CP 2 EpgDocument26 pagesCP 2 EpgMohammad BasitNo ratings yet
- 12.thermodynamics MCQDocument47 pages12.thermodynamics MCQDebangshi NahaNo ratings yet
- Ethanolamines: Product InformationDocument48 pagesEthanolamines: Product InformationElias0% (1)
- Science 5 ExamDocument4 pagesScience 5 ExamAda Marielle SamaniegoNo ratings yet
- POGGIO - 2016 - Modelling The Anaerobic Digestion of Solid Organic Waste - SubstrateDocument15 pagesPOGGIO - 2016 - Modelling The Anaerobic Digestion of Solid Organic Waste - SubstrateThobiasNo ratings yet
- Es 1176-7 - 2005Document21 pagesEs 1176-7 - 2005Addisu PetrosNo ratings yet