Professional Documents
Culture Documents
How Do Catalysts Work
Uploaded by
ishraqtazishraqOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
How Do Catalysts Work
Uploaded by
ishraqtazishraqCopyright:
Available Formats
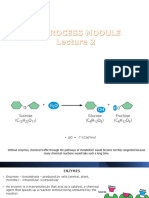
how do catalysts work
Catalysts are substances that increase the rate of a chemical reaction by providing an alternative
pathway with a lower activation energy. The basic principle behind catalysts involves facilitating the
reaction without being consumed or undergoing a permanent change themselves. Here's a more
detailed explanation of how catalysts work:
### 1. **Lowering Activation Energy:**
- Catalysts work by lowering the activation energy required for a chemical reaction to occur. Activation
energy is the minimum energy input required to initiate a reaction. By providing an alternative reaction
pathway with a lower activation energy, catalysts make it easier for the reactants to overcome the
energy barrier and transform into products.
### 2. **Formation of Intermediate Complexes:**
- Catalysts often form temporary intermediate complexes with the reactants. These complexes have
different structures than the reactants and products, and they allow the reaction to proceed more
readily. The formation of these complexes stabilizes the transition state, reducing the overall energy
barrier.
### 3. **Changing Reaction Mechanism:**
- Catalysts can change the mechanism of a reaction, introducing new intermediate steps that are more
favorable energetically. This altered pathway may involve breaking and forming different chemical
bonds, ultimately leading to the same products as the uncatalyzed reaction but through a more efficient
route.
### 4. **Providing an Alternative Reaction Pathway:**
- Catalysts provide an alternative route for the reaction by creating a different sequence of steps. This
alternative pathway often involves more favorable intermediate states, allowing the reaction to proceed
more rapidly.
### 5. **Increasing Collision Frequency:**
- Catalysts can also increase the frequency of collisions between reactant molecules. Higher collision
frequency increases the chances of successful collisions that lead to product formation.
### 6. **Regeneration of Catalysts:**
- Importantly, catalysts are not consumed in the reaction; they remain unchanged and are available to
catalyze multiple cycles of the reaction. This property distinguishes them from reactants, which are
typically consumed during a chemical reaction.
### 7. **Types of Catalysts:**
- Catalysts can be classified into two main types: homogeneous and heterogeneous. Homogeneous
catalysts are in the same phase as the reactants, while heterogeneous catalysts are in a different phase.
Enzymes are a biological example of homogeneous catalysts, while transition metal surfaces often act as
heterogeneous catalysts.
### 8. **Specificity:**
- Catalysts can exhibit specificity, meaning they may selectively catalyze a particular reaction or class of
reactions. This specificity is often due to the precise arrangement of functional groups on the catalyst's
surface.
In summary, catalysts work by providing an alternative reaction pathway with a lower activation energy,
facilitating the conversion of reactants into products. They play a crucial role in various industrial
processes, biological systems, and environmental reactions by making these processes more efficient.
You might also like
- Catalyst: CatalysesDocument5 pagesCatalyst: CatalysesDr. savita goyalNo ratings yet
- Enzymes Organic ChemistryDocument6 pagesEnzymes Organic Chemistryمحمد الكبيسيNo ratings yet
- Chap 5 Bio IgcseDocument7 pagesChap 5 Bio Igcseananya.arumugarajanNo ratings yet
- CatalystsDocument2 pagesCatalystsusulasia777No ratings yet
- Physci Catalyst 101Document34 pagesPhysci Catalyst 101Kyuptonite KimNo ratings yet
- General Principles of CatalysisDocument12 pagesGeneral Principles of CatalysisOLUWASEGUN K AfolabiNo ratings yet
- Enzymes - Part IIDocument71 pagesEnzymes - Part IIBarış KaplanNo ratings yet
- Enzymes Tutorial Part 1 and 2Document10 pagesEnzymes Tutorial Part 1 and 2Akeisha King100% (1)
- Enzymes: Contents in Chapter 1Document12 pagesEnzymes: Contents in Chapter 1Alees RahaizanNo ratings yet
- ENZYMESDocument31 pagesENZYMESjuunisai6No ratings yet
- Module 3 Sec 2 v2Document14 pagesModule 3 Sec 2 v2James MagnoNo ratings yet
- CREII-Module-I - Lecture 2Document28 pagesCREII-Module-I - Lecture 2Aditya parasNo ratings yet
- Facts at Your Fingertips: Catalysis FundamentalsDocument1 pageFacts at Your Fingertips: Catalysis Fundamentalsjdgh1986No ratings yet
- 231 Lecture 5Document16 pages231 Lecture 5Rana AbdullahNo ratings yet
- CATALYSIS by Tejas SahooDocument15 pagesCATALYSIS by Tejas Sahootejassahoo123No ratings yet
- 5243 Heterogeneous Catalysis1Document7 pages5243 Heterogeneous Catalysis1Mohit PatelNo ratings yet
- Enzymes and Biological: CatalysisDocument41 pagesEnzymes and Biological: CatalysisKiya AlemuNo ratings yet
- Lectures 2 PDFDocument7 pagesLectures 2 PDFshubhamNo ratings yet
- CREII-Module-I - Lecture 2 PDFDocument28 pagesCREII-Module-I - Lecture 2 PDFshubhamNo ratings yet
- 1FFF11B1BD16627EE05400144FEB5F70.pptDocument63 pages1FFF11B1BD16627EE05400144FEB5F70.pptNur AishaNo ratings yet
- Chemical Product and Process Design: Dr. Aprilina Purbasari, ST, MTDocument32 pagesChemical Product and Process Design: Dr. Aprilina Purbasari, ST, MTnurfadilla raufNo ratings yet
- 06Enz1AMO LICYAYODocument11 pages06Enz1AMO LICYAYOMohamidin MamalapatNo ratings yet
- Enzymes: Function and StructureDocument5 pagesEnzymes: Function and Structuretiyf mojaleedNo ratings yet
- Enzyme: Enzymes AreDocument11 pagesEnzyme: Enzymes ArePiyush BhallaNo ratings yet
- EnzymesDocument205 pagesEnzymes464464naifNo ratings yet
- S.5 EnzymesDocument16 pagesS.5 Enzymesmusokelukia6No ratings yet
- Catalysis: Srujal RanaDocument45 pagesCatalysis: Srujal RanavandanNo ratings yet
- Lecture 2Document18 pagesLecture 2warden tambiNo ratings yet
- Enzymes - Doc Version 1Document7 pagesEnzymes - Doc Version 1LALITH SAI KNo ratings yet
- Materi 2Document34 pagesMateri 2siti purnamaNo ratings yet
- Adiabatic Fixed-Bed Reactors: Practical Guides in Chemical EngineeringFrom EverandAdiabatic Fixed-Bed Reactors: Practical Guides in Chemical EngineeringNo ratings yet
- Sameer Ahmed Group: GM-01: Function and StructureDocument5 pagesSameer Ahmed Group: GM-01: Function and StructureSameer ButtNo ratings yet
- Activity 12 Enzyme ActivityDocument4 pagesActivity 12 Enzyme ActivityEllen May Diaz-ToringNo ratings yet
- Lecture 1 - The Kinetics of Enzyme Catalyzed ReactionsDocument18 pagesLecture 1 - The Kinetics of Enzyme Catalyzed ReactionsGenevive S. de VeraNo ratings yet
- Chem 3Document103 pagesChem 3César ArenasNo ratings yet
- Subject: Overview of Catalytic Reactor DesignDocument5 pagesSubject: Overview of Catalytic Reactor DesignWhite HeartNo ratings yet
- Study On EnzymesDocument21 pagesStudy On EnzymesPragyan Kumar PradhanNo ratings yet
- Biology ProjectDocument41 pagesBiology ProjectGanesan Siva67% (12)
- Enzymes PPT BioDocument22 pagesEnzymes PPT Biovoyav37617No ratings yet
- Mekelle University Ethiopian Institute of Technology-Mekelle Department of Chemical Engineering Process EngineeringDocument77 pagesMekelle University Ethiopian Institute of Technology-Mekelle Department of Chemical Engineering Process EngineeringetayhailuNo ratings yet
- Enzymology: Theresa May Chin TanDocument20 pagesEnzymology: Theresa May Chin TanVejerla PriyankaNo ratings yet
- Biochemistry 1: - Biochemistry of Amino Acids - Biochemistry of Proteins - Portrait of Allosteric ProteinDocument55 pagesBiochemistry 1: - Biochemistry of Amino Acids - Biochemistry of Proteins - Portrait of Allosteric ProteinHiba N IkhmyesNo ratings yet
- 06Enz1AMO SuarezDocument12 pages06Enz1AMO SuarezscasuarezNo ratings yet
- Metabolism (HL)Document26 pagesMetabolism (HL)Sapreen KaurNo ratings yet
- CatalysisDocument50 pagesCatalysisnagendra_rdNo ratings yet
- 1 Reactor Equipment DesignDocument16 pages1 Reactor Equipment DesignZarar SaleemNo ratings yet
- Catalytic Dehydration of Alcohols: University of Warsaw Faculty of Chemistry Chemical Technology DivisionDocument12 pagesCatalytic Dehydration of Alcohols: University of Warsaw Faculty of Chemistry Chemical Technology DivisionSpandan GhoshalNo ratings yet
- Ma2003 Bright Osinachi Ndubuisi - Enzyme Assay, Mechanism of Action and Kinetics of Enzymatic Catalysis.Document3 pagesMa2003 Bright Osinachi Ndubuisi - Enzyme Assay, Mechanism of Action and Kinetics of Enzymatic Catalysis.geddy D.No ratings yet
- Lasaca Report (Enzymes)Document43 pagesLasaca Report (Enzymes)MaryAnn LasacaNo ratings yet
- Enzyme Kinetics Lecture NoteDocument51 pagesEnzyme Kinetics Lecture Notedowndstairs45No ratings yet
- Group 2 - EnzymesDocument3 pagesGroup 2 - Enzymesditucalan.ha2003No ratings yet
- Biology Unit 8.1Document5 pagesBiology Unit 8.1chalihflNo ratings yet
- 3 EnzymesDocument27 pages3 Enzymesharshit khareNo ratings yet
- Fermentation and Biochemical Engineering: Principles and ApplicationsDocument25 pagesFermentation and Biochemical Engineering: Principles and ApplicationsFabianNo ratings yet
- Chapter 1 NewDocument102 pagesChapter 1 NewFakhrulShahrilEzanieNo ratings yet
- Enzymes: Protein CatalystsDocument15 pagesEnzymes: Protein Catalystsgmurthy_1No ratings yet
- Gen - Chem 2 VELASCO ENCARNACION Part 2Document12 pagesGen - Chem 2 VELASCO ENCARNACION Part 2Edreyan Adong Cortez LimbagaNo ratings yet
- Bio201 Lecture 12 Enzymes - Energy & MetabolismDocument34 pagesBio201 Lecture 12 Enzymes - Energy & MetabolismPaulNo ratings yet
- Slide EnzymeDocument27 pagesSlide EnzymeReni Fatmawati100% (1)
- Contemporary Catalysis: Fundamentals and Current ApplicationsFrom EverandContemporary Catalysis: Fundamentals and Current ApplicationsNo ratings yet
- Cosmology Questions and Answers - SanfoundryDocument9 pagesCosmology Questions and Answers - SanfoundryGopinathan MNo ratings yet
- Maxdb Backup RecoveryDocument44 pagesMaxdb Backup Recoveryft1ft1No ratings yet
- Letter For LACDocument7 pagesLetter For LACDahlia G. MaglasangNo ratings yet
- Python Versus Matlab: Examples in Civil EngineeringDocument32 pagesPython Versus Matlab: Examples in Civil EngineeringNiranjanAryan100% (1)
- PostmanPat Activity PackDocument5 pagesPostmanPat Activity PackCorto Maltese100% (1)
- Wec14 01 Rms 20230112Document23 pagesWec14 01 Rms 20230112Shafay SheikhNo ratings yet
- Edwards SVV HandoutDocument2 pagesEdwards SVV HandoutossinNo ratings yet
- Designing The Workplace For CollaborationDocument17 pagesDesigning The Workplace For Collaborationmas zak danielNo ratings yet
- Defining The Market Research Problem & Developing An ApproachDocument77 pagesDefining The Market Research Problem & Developing An ApproachSakshi Bhati I H21O41No ratings yet
- Symbolic Interaction Theory: Nilgun Aksan, Buket Kısac, Mufit Aydın, Sumeyra DemirbukenDocument3 pagesSymbolic Interaction Theory: Nilgun Aksan, Buket Kısac, Mufit Aydın, Sumeyra DemirbukenIgor Dutra BaptistaNo ratings yet
- Lier Upper Secondary SchoolDocument8 pagesLier Upper Secondary SchoolIES Río CabeNo ratings yet
- Hurricanes Reading Comprehension FreebieDocument20 pagesHurricanes Reading Comprehension FreebieAlex WaddellNo ratings yet
- Internet in My LifeDocument4 pagesInternet in My LifeАндріана ПрусNo ratings yet
- Empowerment TechnologyDocument2 pagesEmpowerment TechnologyRegina Mambaje Alferez100% (1)
- Algorithm Design TechniquesDocument24 pagesAlgorithm Design TechniquespermasaNo ratings yet
- Cot 4 Mapeh (Health)Document15 pagesCot 4 Mapeh (Health)RELYN LUCIDONo ratings yet
- Emergency Floatation Helicoptero PDFDocument14 pagesEmergency Floatation Helicoptero PDFterrywhizardhotmail.com The Best Of The Best.No ratings yet
- PWC - Digital Pocket Tax Book 2023 - SlovakiaDocument52 pagesPWC - Digital Pocket Tax Book 2023 - SlovakiaRoman SlovinecNo ratings yet
- Pro Con ChartDocument3 pagesPro Con Chartapi-461614875No ratings yet
- AMST 398 SyllabusDocument7 pagesAMST 398 SyllabusNatNo ratings yet
- Mooka Panchsati Arya SatakamDocument18 pagesMooka Panchsati Arya SatakamPrasad Raviprolu100% (1)
- 1973 Essays On The Sources For Chinese History CanberraDocument392 pages1973 Essays On The Sources For Chinese History CanberraChanna LiNo ratings yet
- POLAR BEARS-Biology ProjectDocument16 pagesPOLAR BEARS-Biology Projectserwaa21No ratings yet
- Computer Forensics ReportDocument7 pagesComputer Forensics ReportMatias IacobuzioNo ratings yet
- E Catalog YooilDocument10 pagesE Catalog Yooilom jangidNo ratings yet
- Part-II Poem Article and Report For College Magazine-2015-16 Dr.M.Q. KhanDocument4 pagesPart-II Poem Article and Report For College Magazine-2015-16 Dr.M.Q. KhanTechi Son taraNo ratings yet
- Vacuum Braking SystemDocument20 pagesVacuum Braking SystemPrashant RaiNo ratings yet
- CSMP77: en Es FRDocument38 pagesCSMP77: en Es FRGerson FelipeNo ratings yet
- MikroekonomiDocument1 pageMikroekonomiYudhaPrakosoIINo ratings yet
- Clay & Shale Industries in OntarioDocument193 pagesClay & Shale Industries in OntarioJohn JohnsonNo ratings yet