Professional Documents
Culture Documents
Đề 2
Đề 2
Uploaded by
Phạm Minh Cường0 ratings0% found this document useful (0 votes)
5 views1 pageThe document is a test with questions about the phase diagram for the solid-liquid equilibrium of a silver-copper binary mixture. It includes a figure of the phase diagram. The questions ask to:
1) Identify the eutectic point on the graph and state its composition.
2) Determine the maximum amount of copper that can be present in a solid silver phase.
3) For two mixtures at 900°C, identify the present phases, give the composition of each phase, and state the mass of silver and copper in each phase.
Original Description:
its is really good when u read it
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document is a test with questions about the phase diagram for the solid-liquid equilibrium of a silver-copper binary mixture. It includes a figure of the phase diagram. The questions ask to:
1) Identify the eutectic point on the graph and state its composition.
2) Determine the maximum amount of copper that can be present in a solid silver phase.
3) For two mixtures at 900°C, identify the present phases, give the composition of each phase, and state the mass of silver and copper in each phase.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
5 views1 pageĐề 2
Đề 2
Uploaded by
Phạm Minh CườngThe document is a test with questions about the phase diagram for the solid-liquid equilibrium of a silver-copper binary mixture. It includes a figure of the phase diagram. The questions ask to:
1) Identify the eutectic point on the graph and state its composition.
2) Determine the maximum amount of copper that can be present in a solid silver phase.
3) For two mixtures at 900°C, identify the present phases, give the composition of each phase, and state the mass of silver and copper in each phase.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
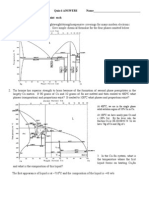
BÀI KIỂM TRA – ĐỀ 2
1. A phase diagram for the solid liquid equilibrium of a binary mixture of silver(Ag)
and copper(Cu) is show below. Answer the following question. Note that the weight
percentage is on the bottom and the mole percentage is on the top.
a. On the graph, indentify the Eutecti. What is the composition of the Eutecti?
b. What is most copper that can be present in a phase of solid Silver?
c. Consider a mixture of 8g silver and 2g copper at 900oC
c1. What phase or phases are present?
c2. What is the composition of each phase?
c3. How many gams silver and copper in each phase?
d. Consider a mixture of 4g silver and 16g copper at 900oC
c1. What phase or phases are present?
c2. What is the composition of each phase?
c3. How many gams silver and copper in each phase?
You might also like
- TYPD ExercisesDocument10 pagesTYPD ExercisesConstance Lynn'da GNo ratings yet
- 02 Phase Diagram of AlloysDocument60 pages02 Phase Diagram of AlloysBudi HerwantoNo ratings yet
- WBJEE 2015 Chemistry Question Answerkey SolutionsDocument21 pagesWBJEE 2015 Chemistry Question Answerkey SolutionsANIKET ROYNo ratings yet
- ENSC 3313, Fall 2012 Quiz 6 Answers Name - Answer Any 4 Questions Only, 1 Point EachDocument2 pagesENSC 3313, Fall 2012 Quiz 6 Answers Name - Answer Any 4 Questions Only, 1 Point EachterratempestNo ratings yet
- PSet 8 FL13Document5 pagesPSet 8 FL13cacer009No ratings yet
- Ap Unit10 WorksheetDocument4 pagesAp Unit10 Worksheetburcak gecNo ratings yet
- Chapter 4 ElectrolysisDocument8 pagesChapter 4 ElectrolysisPremNo ratings yet
- Chem-TEST: Name: - Class: - DateDocument6 pagesChem-TEST: Name: - Class: - DatesdcccdNo ratings yet
- Tutorial 2Document4 pagesTutorial 2maittt.22ba13211No ratings yet
- CHM271 - Tutorial 4 - ElectrochemistryDocument5 pagesCHM271 - Tutorial 4 - Electrochemistrynurfarisha2809No ratings yet
- Practice Problems 7Document15 pagesPractice Problems 7Deena RuangchayNo ratings yet
- 10th Chemistry Revision Assignments - All Chapters CombinedDocument11 pages10th Chemistry Revision Assignments - All Chapters CombinedYash KapoorNo ratings yet
- Homework 5Document4 pagesHomework 5Ferhat PeynirciNo ratings yet
- Chemistry TestDocument4 pagesChemistry TestCarrie PerryNo ratings yet
- Electrochemistry Notes Top ClassDocument13 pagesElectrochemistry Notes Top ClassSriyansh GhoshNo ratings yet
- Chapter N9 Proplems HW - Ch9Document1 pageChapter N9 Proplems HW - Ch9xuantoni1112003No ratings yet
- Actual Repeat Paper 2013Document10 pagesActual Repeat Paper 2013Jasmeet Kaur SandhuNo ratings yet
- Assignment PhaseDiaDocument5 pagesAssignment PhaseDiaAnshu Kumar GuptaNo ratings yet
- Redox and Electrochem Review Multiple Choice Eboard AnswersDocument4 pagesRedox and Electrochem Review Multiple Choice Eboard AnswersKhaledEl-MaghallawyNo ratings yet
- Chemistry Handout 9 REF #: 009: The Mole and Chemical ReactionsDocument3 pagesChemistry Handout 9 REF #: 009: The Mole and Chemical ReactionsNaomi JohnsonNo ratings yet
- 12.1 ExerciseDocument8 pages12.1 ExerciseDakarirayi MutenherwaNo ratings yet
- Pre Trial Sem 2 June 2023Document9 pagesPre Trial Sem 2 June 2023Fazliawati MahayuddinNo ratings yet
- ChemistryDocument2 pagesChemistrypriya yadavNo ratings yet
- Revision-2 - On ElectrochemistryDocument12 pagesRevision-2 - On ElectrochemistryKiro RemonNo ratings yet
- HW 8 TrustedDocument20 pagesHW 8 TrustedEldeniz AliyevNo ratings yet
- Ch2 MCQ PDFDocument6 pagesCh2 MCQ PDFPratibha BhondeNo ratings yet
- Assignment 1: DEADLINE: Monday, 17 January 2022Document10 pagesAssignment 1: DEADLINE: Monday, 17 January 2022Syafiqah RusdiNo ratings yet
- Worksheet Chemo G 12 Unit Tu 22 2016Document9 pagesWorksheet Chemo G 12 Unit Tu 22 2016Dagim YenenehNo ratings yet
- Materials 2 May 2019 - QUESTIONS - PHDocument24 pagesMaterials 2 May 2019 - QUESTIONS - PHmalek1322004No ratings yet
- Ejercicios QuímicaDocument3 pagesEjercicios QuímicaAndreaForteRuizNo ratings yet
- Electroplating WorksheetDocument2 pagesElectroplating WorksheetHồ Thị Ngọc MinhNo ratings yet
- 9.1. Consider T-WPS OfficeDocument4 pages9.1. Consider T-WPS OfficeTia AgustinNo ratings yet
- Closed-Book Practice-Ch 09 (2017!08!07)Document9 pagesClosed-Book Practice-Ch 09 (2017!08!07)Juan100% (1)
- Tutorial 3 ElectrochemistryDocument6 pagesTutorial 3 ElectrochemistrymunirahNo ratings yet
- Reduction-Oxidation Reactions and ElectrochemistryDocument14 pagesReduction-Oxidation Reactions and Electrochemistrykaushi123No ratings yet
- Latihan Ujian Sekolah KimiaDocument3 pagesLatihan Ujian Sekolah Kimiarosyad ZulfikarNo ratings yet
- Transition Metals H2 QuestionsDocument7 pagesTransition Metals H2 QuestionskitoniumNo ratings yet
- Redox QuizzesDocument9 pagesRedox Quizzesoscarball06No ratings yet
- Thermoq 2Document4 pagesThermoq 2bobNo ratings yet
- Electrochemistry Assignment-2Document2 pagesElectrochemistry Assignment-2Akshara SreeNo ratings yet
- 12 Question BankDocument50 pages12 Question BankAbhiNo ratings yet
- Topic 3/13 Practice IB Chem TestDocument12 pagesTopic 3/13 Practice IB Chem TestKeyerria HowardNo ratings yet
- Electrochemical Reactions: + Battery - Salt BridgeDocument7 pagesElectrochemical Reactions: + Battery - Salt BridgewscienceNo ratings yet
- CHEM101 172 Final SolvedDocument12 pagesCHEM101 172 Final SolvedTorong VNo ratings yet
- CHEM455 2021 Problem Set 2Document3 pagesCHEM455 2021 Problem Set 2Hae Eun JeongNo ratings yet
- Question Bank Chemistry XI Term - 2Document4 pagesQuestion Bank Chemistry XI Term - 2GHOSTX GAMERNo ratings yet
- Tutorial 2 HelperDocument3 pagesTutorial 2 HelperDedy SaputraNo ratings yet
- Tutorial Problems 5Document8 pagesTutorial Problems 5Majak MarialNo ratings yet
- Oxidation-Reduction p4Document6 pagesOxidation-Reduction p4maramalhaj404No ratings yet
- Set 4 Practice To A QuestionDocument8 pagesSet 4 Practice To A QuestionaNo ratings yet
- MC Practice 2Document10 pagesMC Practice 2jackson wongNo ratings yet
- 9A03301 Materials Science and EngineeringDocument4 pages9A03301 Materials Science and EngineeringsivabharathamurthyNo ratings yet
- Chapter 1: Structure: Universiti Teknologi MaraDocument16 pagesChapter 1: Structure: Universiti Teknologi MaraRasyidi AhmadNo ratings yet
- Grade 9 Term 1 Chemistry RevisionDocument7 pagesGrade 9 Term 1 Chemistry Revisionsiddloves.snowNo ratings yet
- Electron Config and Molar MassDocument1 pageElectron Config and Molar MassLinden EvangelistaNo ratings yet
- Physics and Technology of Crystalline Oxide Semiconductor CAAC-IGZO: FundamentalsFrom EverandPhysics and Technology of Crystalline Oxide Semiconductor CAAC-IGZO: FundamentalsNo ratings yet