Professional Documents
Culture Documents
Ap Chem
Uploaded by
holaholaholacarlottaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ap Chem
Uploaded by
holaholaholacarlottaCopyright:
Available Formats
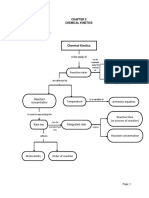
NOTES:
RATE LAW USING INITIAL CONCENTRATIONS:
rate law: The dependence of the initial rate of a reaction on the concentrations of its reactant
can't
*
be determined from balanced equation, it must be determined from
experimental
data.
the greater reactants
exponent
the value of a
the more a change in the concentration of
(83 "[C]=
*
Rate = k [A]
that reactant will affect the rate of reaction
to find values of exponents
x, y, and
2, we need to find how changes
in the individual reactants affect the rate.
the easiest way to find the
exports is to see what happens to the rate when the concentration
oan individual reactant is doubled
RATE LAW USINO CONCENTRATION AND TIME:
rate laws will be different
depending on wether the reaction is first,
second, or zero order, but each rate law can be expressed
as graph that relates the rate
a
constant, the concentration of a reactant
and the elapsed time.
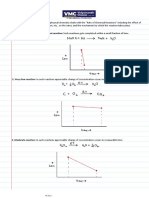
ZERO OROER RATE LAWS:
does not depend on the concentration of reactants at all, so it
will always be the same at
given temperature: Rate = k
FIRST ORDER RATE LANS:
the rate of the first order reaction depends on the concentration of a
single reactant raised to the first power. Rate = kCA]
First order rate law: In [A]t=-k++ InCAJO
CAT+= concentration of reactant A at time t #= rate constant
CAT0 = initial concentration of reactant A += time elapsed
SECONO-OROER RATE LAWS:
depends on the concentration of a single reactant raised to second power.
ate
*
= kCA]2
it uses the
*
inverses of the concentrations.
(A) 1
k
=
+ +
EATo
[ ]+= concentration of reactant A
CAJo = initial concentration
at time
of reactant
t
A
K = rate constant t =
time elapsed
HALF-LIFE:
The half-life of a reactant in a chemical reaction is the time it takes
for half of the substance to react
For order second order
reactants, half-life NOT
*
zero or is
constant. You cant use the half life equation for anything other
↓ han a first-order reactant
COLLISION THEORY:
According collision chemical
to
theory,
reactions occur because reactents
are constantly moving around and
colliding with one another
reaction
* rates increase with
increasing temperature because
increasing temperature means
that the molecules are
moving
faster, which means that the
molecules have
greater Kinetic energy. The higher the
average
temp, the greater the number of reactant molecules colliding with
each other with enough energy (Ea) to cause a reaction
* EER's LAW:
to measure the concentration of a solution over
time, a device
called a spectrophotometer can be used in some situations.
It measures the amount of light at a
given wavelength that
is absorbed by a solution. Absorbance can be calculated
using Geer's law: A ab <
=
A =
absorbance, a molar
absorptivity, b = bath length
c concentration of the solution
CATALYSTS:
catalysts increase the rate of a chemical
reaction without the
being consumed in
the
process; catalysts don't appear in
balanced
equation. Some times
they are
necessary in a reaction because in its
absence, the reaction
would be too slow to be useful,
18
MCQs (313-318) 1, 3, 5, 7, 8, 11, 13,15 FRQs(319-327)
cancel out.
1:a +
y
3: H: C+A 7P 5: D
C +* -> 0 half life of
A+ 8 + A58 (amount
8x -> y+E timetaken to dealers
2A + 8- 8
2A +C7E concentratious temperateene
⑳ *
Rate = k(A]([B] affect half life
7.the slowest matches 8: B
the overall rate
law
ENO)1 -> ENO+12
but instead all,
-
of 240 molecules that
2
every form,
it should be substituted NOCI dissappear.
by 2A.8
11.c because if you look at the two CA) (M) stays the
experiments,
same in the first and second experiment, while (B(M)
doubles from experiment. The rate
doubles
first to second
which means & affects rate law. In experiment and
3 the and A
it was
opposite, doubled while 8 stayed
the same. The rate didnt change when A wasdoubled.
t his means the rate law is c=k(8) because it
showed with more
impact in
quantity,
on rate
13: In the 1st and and experiment, when Only 8 is
doubled
the 3rd
rate doubles. In the end and experiments
when both A and B are doubled, the rate increases
*
4. This shows how both A and 8 are important
for the
rate,
which means the rate law is
xCA358]
You might also like
- SY - PP II - Drug StabilityDocument49 pagesSY - PP II - Drug StabilityKevalNo ratings yet
- Chemical Kinetics ReviewerDocument4 pagesChemical Kinetics ReviewervincentnagacNo ratings yet
- Chemical KineticsDocument21 pagesChemical Kineticsdipankargh48No ratings yet
- Gen Chem ReviewerDocument9 pagesGen Chem ReviewerMalayao, Philip Jude M.No ratings yet
- Chemical KineticsDocument9 pagesChemical KineticsMikey Bryant BonbonNo ratings yet
- Chemistry Notes For Class 12 Chapter 4 Chemical KineticsDocument11 pagesChemistry Notes For Class 12 Chapter 4 Chemical KineticsAyush singh PrinceNo ratings yet
- XII - CHEMICAL KINETICS - Module 2Document5 pagesXII - CHEMICAL KINETICS - Module 2Rahul Joseph ThomasNo ratings yet
- Revision Questions Chapter 4 Chemical KineticsDocument23 pagesRevision Questions Chapter 4 Chemical Kineticssimple student akashNo ratings yet
- Group 4 - Chemical KineticsDocument54 pagesGroup 4 - Chemical KineticsMark Harold GonzalesNo ratings yet
- Chapter 4 Gen. Chem 2Document29 pagesChapter 4 Gen. Chem 2John Victor MalupaNo ratings yet
- Chemical KineticsDocument34 pagesChemical Kineticskingrustam950No ratings yet
- Xii Iit Chemistry-Chemical Kinetics-Rate Equation CW-1: Types of Rates of Chemical ReactionDocument8 pagesXii Iit Chemistry-Chemical Kinetics-Rate Equation CW-1: Types of Rates of Chemical ReactionRsrao JNo ratings yet
- Class XII Chemistry Ch. 4: Chemical Kinetics Chapter Notes Key LearningsDocument5 pagesClass XII Chemistry Ch. 4: Chemical Kinetics Chapter Notes Key LearningsramjuriyaNo ratings yet
- Determining A Rate Law EquationDocument8 pagesDetermining A Rate Law EquationMark ButlerNo ratings yet
- Chapter 3 Chemical KineticsDocument46 pagesChapter 3 Chemical KineticsaadarshceoNo ratings yet
- Kuliah Teknik Reaksi Kimia HomogenDocument34 pagesKuliah Teknik Reaksi Kimia HomogenThe Golden PieNo ratings yet
- Bab 2 - Kinetika Reaksi HomogenDocument12 pagesBab 2 - Kinetika Reaksi HomogenDiah Ayu TriatNo ratings yet
- Chemical Kinetics - NotesDocument6 pagesChemical Kinetics - Notesn611704No ratings yet
- Kinetics Mastery AnswersDocument5 pagesKinetics Mastery AnswersAnonymous vRpzQ2BLNo ratings yet
- Chemical Kinetics: By:-Divyam Verma Ankur Kumar Deepak KumarDocument36 pagesChemical Kinetics: By:-Divyam Verma Ankur Kumar Deepak KumarAnindya BhattacharyaNo ratings yet
- Chemical Kinetics Study Material & QuestionsDocument26 pagesChemical Kinetics Study Material & QuestionsKRITHIKA .MNo ratings yet
- Chemical Kinetics and ColloidsDocument6 pagesChemical Kinetics and Colloidstahasheikh822No ratings yet
- Chemical KineticsDocument29 pagesChemical KineticsManohar MaripeNo ratings yet
- Rate of Reactions: Abbey Jimenez & Aimee RollorataDocument10 pagesRate of Reactions: Abbey Jimenez & Aimee Rollorataroldan rollorataNo ratings yet
- Chapter 9Document18 pagesChapter 9JeromeNo ratings yet
- Chemical Kinetics-1 NotesDocument14 pagesChemical Kinetics-1 NotesRachit rajputNo ratings yet
- Chemical KineticsDocument29 pagesChemical KineticsAditya PandeyNo ratings yet
- Chemical KineticsDocument1 pageChemical KineticsSachinNo ratings yet
- Chemical Kinetics TheoryDocument31 pagesChemical Kinetics TheoryKivilia EduventuresNo ratings yet
- Chapter 3 PART1-Rate LawsDocument32 pagesChapter 3 PART1-Rate Laws林哲璋No ratings yet
- Chemical Kinetics: Gist of The LessonDocument34 pagesChemical Kinetics: Gist of The Lessonanshikahp1No ratings yet
- Chemical KineticsDocument51 pagesChemical KineticsSrynnENo ratings yet
- CBSE Class 12 Chemistry Notes: Chemical Kinetics: HomepageDocument14 pagesCBSE Class 12 Chemistry Notes: Chemical Kinetics: HomepageBHAVYA BNo ratings yet
- Chemistry Unit 4 PDFDocument60 pagesChemistry Unit 4 PDFsammam mahdi samiNo ratings yet
- Chemical KineticsDocument28 pagesChemical KineticsNavu SinghNo ratings yet
- 3-Law of Mass ActionDocument10 pages3-Law of Mass ActionLiluNo ratings yet
- Kinetics LPDocument41 pagesKinetics LPHarkritSinghNo ratings yet
- 4 - The Rates of Chemical Reactions - ADocument42 pages4 - The Rates of Chemical Reactions - AÇetin UzişNo ratings yet
- WastedDocument9 pagesWastedMark Francis DaculanNo ratings yet
- 21 Chemical Kinetics Formula Sheets Getmarks AppDocument8 pages21 Chemical Kinetics Formula Sheets Getmarks AppRockstarNo ratings yet
- Chemical KineticDocument40 pagesChemical KineticHamzaNo ratings yet
- Chemical Kinetics PDFDocument42 pagesChemical Kinetics PDFrockingrazzNo ratings yet
- This PDF Is The Sample PDF Taken From Our Comprehensive Study Material For IIT-JEE Main & AdvancedDocument12 pagesThis PDF Is The Sample PDF Taken From Our Comprehensive Study Material For IIT-JEE Main & AdvancedGod is every whereNo ratings yet
- Surface Chemistry CH NotesDocument5 pagesSurface Chemistry CH NotesSanvi ShanNo ratings yet
- Reactor Chapter1Document9 pagesReactor Chapter1Sisanda MakhalimaNo ratings yet
- Chemical KineticszzDocument29 pagesChemical KineticszzfailurewasteworthlessNo ratings yet
- Lecture (4) : Reaction RatesDocument21 pagesLecture (4) : Reaction RatesAhmed AbdullaNo ratings yet
- Lecture 4a. Chemical Kinetics 2020Document23 pagesLecture 4a. Chemical Kinetics 2020Montassar DridiNo ratings yet
- Kinetics & Equilibrium NotesDocument30 pagesKinetics & Equilibrium Notesfirebot4No ratings yet
- Edexcel International A Level Chemistry Unit 4 Rates Equilibria and Further Organic Chemistry wch1401 v6Document9 pagesEdexcel International A Level Chemistry Unit 4 Rates Equilibria and Further Organic Chemistry wch1401 v6emdyoverdriveNo ratings yet
- Chemical Kinetics Neet MCQDocument12 pagesChemical Kinetics Neet MCQmanan10jas1529No ratings yet
- Xii - CH4 - Chemical KineticsDocument3 pagesXii - CH4 - Chemical KineticsYash RajNo ratings yet
- Kinetics of Homogeneous ReactionsDocument13 pagesKinetics of Homogeneous ReactionsRahul ParmarNo ratings yet
- Chemical Kinetics PDFDocument9 pagesChemical Kinetics PDFPriyanshu amanNo ratings yet
- Chemical Kinetics: The Rates and Mechanisms of Chemical ReactionsDocument21 pagesChemical Kinetics: The Rates and Mechanisms of Chemical ReactionsOyinkansola OsiboduNo ratings yet
- Kinetics NotesDocument18 pagesKinetics NotesAnuki PereraNo ratings yet
- Chemical KineticsDocument40 pagesChemical KineticsHirdesh Sehgal100% (3)
- Chemical Kinetics TheoryDocument30 pagesChemical Kinetics TheoryBichitra GautamNo ratings yet
- Book 2Document424 pagesBook 2Anonymous DPt7hpiaNo ratings yet
- Sealants Product List by Specification Custodian PDFDocument29 pagesSealants Product List by Specification Custodian PDFDimas PratamaNo ratings yet
- TARSONS Export Catalogue 2010Document56 pagesTARSONS Export Catalogue 2010fraud12345No ratings yet
- Ultrasonic Cleaner GuideDocument13 pagesUltrasonic Cleaner GuideDCG CandyNo ratings yet
- Case Study of Toluene HDA - DouglasDocument42 pagesCase Study of Toluene HDA - DouglasSurenthran Sundar50% (2)
- Chou - Structure and Properties of Composites (1993) PDFDocument603 pagesChou - Structure and Properties of Composites (1993) PDFjoereisNo ratings yet
- SaleSheets AirPure AirPurifier enDocument2 pagesSaleSheets AirPure AirPurifier enMmt RdcNo ratings yet
- Simmering CombiDocument16 pagesSimmering Combicosta59dac9242No ratings yet
- Pharmacognosy and Plant ChemistryDocument11 pagesPharmacognosy and Plant ChemistryKaithlyn ObispoNo ratings yet
- Fluid Velocity in PipesDocument1 pageFluid Velocity in PipesDan SabadusNo ratings yet
- Oxford EDS - Silicon Drift Detectors ExplainedDocument28 pagesOxford EDS - Silicon Drift Detectors Explainedrichard addo sowahNo ratings yet
- CAM72FI Final PDFDocument1 pageCAM72FI Final PDFAshikBalaramNo ratings yet
- Review of Coagulation's Rapid Mixing For NOM Removal: Research ArticleDocument32 pagesReview of Coagulation's Rapid Mixing For NOM Removal: Research ArticleMd. Rashedul IslamNo ratings yet
- Introduction To Materials Characterization PDFDocument26 pagesIntroduction To Materials Characterization PDFMustafa Abbas Mustafa67% (3)
- Assignment SolutionsDocument10 pagesAssignment SolutionsArrianne Jaye MataNo ratings yet
- Agricultural Lime and Liming, Part 3: Aglime Product Selection and Comparison Calculator User GuideDocument11 pagesAgricultural Lime and Liming, Part 3: Aglime Product Selection and Comparison Calculator User GuideDesu MihretuNo ratings yet
- InTech-Mass and Heat Transfer During Thin Film Evaporation of Liquid SolutionsDocument17 pagesInTech-Mass and Heat Transfer During Thin Film Evaporation of Liquid SolutionsClarence AG YueNo ratings yet
- KK5701 PDFDocument4 pagesKK5701 PDFcommgmailNo ratings yet
- Submerged Arc Welding (Saw)Document5 pagesSubmerged Arc Welding (Saw)Nnaji Chukwuma SlamNo ratings yet
- Hydrogen and Fuel Cell Technology (Vafa Chiragova)Document21 pagesHydrogen and Fuel Cell Technology (Vafa Chiragova)Vəfa ÇıraqovaNo ratings yet
- Eldan RecyclingDocument10 pagesEldan RecyclingIvan BrcelicNo ratings yet
- Lecture Outline in Biochemistry Chapter IDocument3 pagesLecture Outline in Biochemistry Chapter IWeljoy LabbaoNo ratings yet
- Magnesium GluconateDocument2 pagesMagnesium GluconateWanguNo ratings yet
- Techniques in Microbiology ZulaikaDocument66 pagesTechniques in Microbiology ZulaikaSiti RedzuanNo ratings yet
- Termo Fermi SolDocument46 pagesTermo Fermi SolȘtefan RăzvanNo ratings yet
- 980.13 Fructose, Glucose, Lactose, Maltose and Sucrose in Milk ChocolateDocument1 page980.13 Fructose, Glucose, Lactose, Maltose and Sucrose in Milk ChocolateJessica triana pinedaNo ratings yet
- Appendix F - Mechanical DesignDocument37 pagesAppendix F - Mechanical DesignPDPPPMAT0621 Ruhilin Binti NasserNo ratings yet
- Corrosion Monitoring PrimerDocument61 pagesCorrosion Monitoring PrimerShubhodeep SarkarNo ratings yet
- Santrophene InjectionDocument27 pagesSantrophene InjectionalfiharadisNo ratings yet
- Biology 25: Human Biology: Prof. Gonsalves Los Angeles City College Loosely Based On Mader's Human Biology, 7 EditionDocument119 pagesBiology 25: Human Biology: Prof. Gonsalves Los Angeles City College Loosely Based On Mader's Human Biology, 7 EditionRhaine EstebanNo ratings yet