Professional Documents
Culture Documents
Jamaneurology Costoyasnchez 2023 Oi 230055 1691685948.4125
Jamaneurology Costoyasnchez 2023 Oi 230055 1691685948.4125
Uploaded by
Yenny MaharaniOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Jamaneurology Costoyasnchez 2023 Oi 230055 1691685948.4125
Jamaneurology Costoyasnchez 2023 Oi 230055 1691685948.4125
Uploaded by
Yenny MaharaniCopyright:
Available Formats
Research
JAMA Neurology | Original Investigation
Increased Medial Temporal Tau Positron Emission
Tomography Uptake in the Absence of Amyloid-β Positivity
Alejandro Costoya-Sánchez, MSc; Alexis Moscoso, PhD; Jesús Silva-Rodríguez, PhD; Michael J. Pontecorvo, PhD; Michael D. Devous Sr, PhD;
Pablo Aguiar, PhD; Michael Schöll, PhD; Michel J. Grothe, PhD; for the Alzheimer’s Disease Neuroimaging Initiative and the Harvard Aging Brain Study
Editorial
IMPORTANCE An increased tau positron emission tomography (PET) signal in the medial Supplemental content
temporal lobe (MTL) has been observed in older individuals in the absence of amyloid-β (Aβ)
pathology. Little is known about the longitudinal course of this condition, and its association
with Alzheimer disease (AD) remains unclear.
OBJECTIVE To study the pathologic and clinical course of older individuals with PET-evidenced
MTL tau deposition (TMTL+) in the absence of Aβ pathology (A−), and the association of this
condition with the AD continuum.
DESIGN, SETTING, AND PARTICIPANTS A multicentric, observational, longitudinal cohort study
was conducted using pooled data from the Alzheimer’s Disease Neuroimaging Initiative
(ADNI), Harvard Aging Brain Study (HABS), and the AVID-A05 study, collected between
July 2, 2015, and August 23, 2021. Participants in the ADNI, HABS, and AVID-A05 studies
(N = 1093) with varying degrees of cognitive performance were deemed eligible if they had
available tau PET, Aβ PET, and magnetic resonance imaging scans at baseline. Of these,
128 participants did not meet inclusion criteria based on Aβ PET and tau PET biomarker
profiles (A+ TMTL−).
EXPOSURES Tau and Aβ PET, magnetic resonance imaging, cerebrospinal fluid biomarkers,
and cognitive assessments.
MAIN OUTCOMES AND MEASURES Cross-sectional and longitudinal measures for tau and Aβ
PET, cortical atrophy, cognitive scores, and core AD cerebrospinal fluid biomarkers (Aβ42/40
and tau phosphorylated at threonine 181 p-tau181 available in a subset).
RESULTS Among the 965 individuals included in the study, 503 were women (52.1%) and the
mean (SD) age was 73.9 (8.1) years. A total of 51% of A− individuals and 78% of A+ participants
had increased tau PET signal in the entorhinal cortex (TMTL+) compared with healthy
younger (aged <39 years) controls. Compared with A− TMTL−, A− TMTL+ participants showed
statistically significant, albeit moderate, longitudinal (mean [SD], 1.83 [0.84] years) tau PET
increases that were largely limited to the temporal lobe, whereas those with A+ TMTL+
showed faster and more cortically widespread tau PET increases. In contrast to participants
with A+ TMTL+, those with A− TMTL+ did not show any noticeable Aβ accumulation over
follow-up (mean [SD], 2.36 [0.76] years). Complementary cerebrospinal fluid analysis
confirmed longitudinal p-tau181 increases in A− TMTL+ in the absence of increased Aβ
accumulation. Participants with A− TMTL+ had accelerated MTL atrophy, whereas those with
A+ TMTL+ showed accelerated atrophy in widespread temporoparietal brain regions. Author Affiliations: Author
Increased MTL tau PET uptake in A− individuals was associated with cognitive decline, affiliations are listed at the end of this
article.
but at a significantly slower rate compared with A+ TMTL+.
Group Information: Members of the
CONCLUSIONS AND RELEVANCE In this study, individuals with A− TMTL+ exhibited progressive Alzheimer’s Disease Neuroimaging
Initiative and the Harvard Aging Brain
tau accumulation and neurodegeneration, but these processes were comparably slow,
Study appear in Supplement 2.
remained largely restricted to the MTL, were associated with only subtle changes in global
Corresponding Authors: Michel J.
cognitive performance, and were not accompanied by detectable accumulation of Aβ Grothe, PhD, Unidad de Trastornos
biomarkers. These data suggest that individuals with A− TMTL+ are not on a pathologic del Movimiento, Instituto de
trajectory toward AD. Biomedicina de Sevilla (IBiS),
Campus Hospital Universitario
Virgen del Rocío, Avda. Manuel
Siurot, s/n, 41013 Sevilla, Spain
(mgrothe@us.es); Michael Schöll,
PhD, Sahlgrenska University Hospital,
Wallinsgatan 6, 43141 Mölndal,
JAMA Neurol. doi:10.1001/jamaneurol.2023.2560 Sweden (michael.scholl@
Published online August 14, 2023. neuro.gu.se).
(Reprinted) E1
Downloaded From: https://jamanetwork.com/ on 08/29/2023
Research Original Investigation Increased Medial Temporal Tau Uptake Without Amyloid-β Positivity
A
myloid-β (Aβ) plaques and tau neurofibrillary tangles
are the hallmarks of Alzheimer disease (AD).1,2 The pres- Key Points
ence of neurofibrillary tangles has been observed to be
Question What is the longitudinal trajectory of older individuals
tightly linked to increased Aβ load.3 However, the presence of who show positron emission tomography–assessed medial
neurofibrillary tangles in the medial temporal lobe (MTL) has temporal lobe (MTL) tau deposition in the absence of amyloid-β
also been observed in older individuals without substantial Aβ (Aβ) pathology (A− TMTL+)?
pathology,4 a condition that has been termed primary age-
Findings In this cohort study of 969 older participants,
related tauopathy (PART).5 Over recent years, clinicopatho- A− TMTL+ individuals displayed moderate tau accumulation
logic association studies have shed light on the clinical and mainly restricted to the MTL, which was paralleled by
neurodegenerative correlates of PART.6-10 Yet, being a neuro- cerebrospinal fluid phosphorylated tau increases and
pathologic entity that is only diagnosed at autopsy, little is colocalized atrophy progression; no significant Aβ accumulation
known about the temporal course of this condition, and its was observed. By contrast, Aβ-positive individuals showed
pronounced and cortically widespread tau accumulation,
association with downstream Aβ accumulation and the AD
which was accompanied by extratemporal cortical atrophy
continuum11 remains controversial.12-14 and significantly faster cognitive decline.
Positron emission tomography (PET) studies have also
consistently shown increased MTL tau PET signal in a subset Meaning The findings of this study suggest that individuals
with A− TMTL+ do not appear to be on a pathologic trajectory
of individuals with negative Aβ PET scans,15-17 which may re-
toward Alzheimer disease.
flect PART, among other possible conditions.18 The in vivo
PET-based identification of these individuals also allows
studying their future clinical and pathologic progression. Subsets of the study participants underwent follow-up neu-
In this study, we used data from a large multicohort sample roimaging (mean [SD], 2.36 [0.76] years for Aβ PET and 1.83
to study the longitudinal pathologic characteristics and fu- [0.84] years for tau PET) and cognitive assessments (eMethods
ture clinical course of Aβ PET-negative (A−) individuals who in Supplement 1). Participants’ characteristics are provided in
show increased MTL tau PET signal (TMTL+). Specifically, we the Table.
studied baseline characteristics and longitudinal changes in
cognition, neuroimaging, and cerebrospinal fluid (CSF) bio- Neuroimaging
markers in these individuals and contrasted them to biomarker- Magnetic resonance imaging acquisition details for ADNI,
negative controls as well as to individuals with an AD-typical HABS, and AVID-A05 are reported in the eMethods in Supple-
Aβ- and tau-positive PET profile. ment 1. Magnetic resonance images were segmented with
FreeSurfer, version 7.1.1 and Statistical Parametric Mapping 12
(SPM12, Wellcome Department of Imaging Neuroscience, In-
stitute of Neurology). FreeSurfer-derived regions of interest
Methods (ROI) were merged to generate masks resembling regions af-
Study Design fected by neurofibrillary tangle pathology in Braak stages I/II,
Data used in the preparation of this article were obtained from III/IV, and V/VI (eMethods in Supplement 1).20,21 FreeSurfer-
the Alzheimer’s Disease Neuroimaging Initiative (ADNI), based cortical thickness maps were coregistered to the fsav-
Harvard Aging Brain Study (HABS),19 and AVID-A05 study co- erage template and smoothed with a 2-dimensional isotropic
horts (eMethods in Supplement 1). Informed written consent gaussian filter of 12 mm full width at half maximum.
was obtained from all participants or their corresponding care- PET acquisitions followed study-specific protocols that are
givers. All protocols were approved by each cohort’s respec- detailed in the eMethods in Supplement 1. Tau-PET scans were
tive institutional ethical review board. This study followed the acquired using [18F]flortaucipir (FTP), and Aβ-PET scans were
Strengthening the Reporting of Observational Studies in Epi- acquired using either [18F]florbetapir (ADNI and AVID-A05),
demiology (STROBE) reporting guideline. Data were collected [18F]florbetaben (ADNI), or [11C]Pittsburgh compound B (HABS)
between July 2, 2015, and August 23, 2021. We included all radiotracers. The multicentric PET scans were preprocessed
participants who had undergone concurrent structural using an in-house-developed pipeline that replicated the ADNI
magnetic resonance imaging, Aβ PET, tau PET, and clinical pipeline for PET scanner harmonization. 22,23 Scanner-
evaluation within a 6-month window (N = 1093). Participants specific gaussian filters were applied to each PET image (re-
were further classified into 4 groups according to PET-based gardless of PET imaging modality) to reach a uniform isotro-
Aβ (A) and tau (T) status, as described in the Neuroimaging pic resolution of 8 mm.
section: A− TMTL− (n = 250), A− TMTL+ (n = 264), A+ TMTL+ For FTP-PET scans, region-based voxelwise24 partial vol-
(n = 451), and A+ TMTL− (n = 128). Additionally, a subcohort of ume correction was applied using the PETPVC toolbox25 and
16 healthy younger controls (maximum age, <39 years) with Baker atlas.26 Global standardized uptake value ratio (SUVR)
concurrent magnetic resonance imaging and tau PET scans in Aβ-PET scans was quantified using the centiloid scale27
from the AVID-A05 study was included for the definition of the (eMethods in Supplement 1). In addition, cortical surface SUVR
tau PET positivity threshold. maps were generated for all PET scans using FreeSurfer,28,29
A subset from the ADNI study had baseline and follow-up coregistered to the fsaverage template, and smoothed with a
CSF biomarkers available (described in the CSF Biomarkers 2-dimensional isotropic gaussian filter of 10 mm full width at
section), and all participants had baseline cognitive data. half maximum.
E2 JAMA Neurology Published online August 14, 2023 (Reprinted) jamaneurology.com
Downloaded From: https://jamanetwork.com/ on 08/29/2023
Increased Medial Temporal Tau Uptake Without Amyloid-β Positivity Original Investigation Research
Table. Cohort Characteristics
Characteristic A− TMTL− (n = 250) A− TMTL+ (n = 264) A+ TMTL+ (n = 451)
Study, No. (%)
ADNI 128 (51.2) 178 (67.4) 330 (73.2)
HABS 65 (26.0) 52 (19.7) 36 (8.0)
AVID-A05 57 (22.8) 34 (12.9) 85 (18.8)
Age, mean (SD), y 70.0 (7.8) 74.9 (7.6) 75.6 (8.0)
Gender, No. (%)
Men 108 (43.2) 133 (50.4) 221 (49.0)
Women 142 (56.8) 131 (49.6) 230 (51.0)
Years of education, mean (SD) 15.96 (2.80) 16.73 (2.60) 16.43 (2.47)
APOE-ε4 carrier, No. (%)a 49 (19.9) 45 (18.0) 236 (54.4)
APOE-ε2 carrier, No. (%)a 42 (16.3) 36 (14.4) 19 (4.4)
Cognitive status, No. (%)
CU 189 (75.6) 175 (66.3) 180 (39.9)
MCI 55 (22.0) 71 (26.9) 172 (38.1)
ADD 6 (2.4) 18 (6.8) 99 (22.0)
MMSE score, mean (SD) 28.9 (1.59) 28.6 (1.93) 26.9 (3.60) Abbreviations,
ADAS-Cog 11, Alzheimer’s Disease
CU PACC-3, mean (SD) 0.25 (1.92) −0.17 (2.30) −0.27 (2.23) Assessment Scale–Cognitive
CI ADAS-Cog 11, mean (SD) 9.85 (5.50) 10.3 (5.4) 13.9 (7.4) Subscale; ADD, Alzheimer disease
Baseline biomarkers, mean (SD) dementia; ADNI, Alzheimer’s Disease
Neuroimaging Initiative;
Centiloids −0.72 (7.76) 0.46 (7.89) 68.52 (37.31) APOE, apolipoprotein E;
Braak stages I/II FTP SUVR 1.10 (0.09) 1.42 (0.35) 1.80 (0.56) CI, cognitive impairment;
Braak stages III/IV FTP SUVR 1.19 (0.08) 1.30 (0.11) 1.72 (0.67) CSF, cerebrospinal fluid; CU, cognitive
unimpairment; FTP, [18F]flortaucipir;
Braak stages V/VI FTP SUVR 1.07 (0.08) 1.15 (0.10) 1.37 (0.44) HABS, Harvard Aging Brain Study;
Log CSF Aβ42/40 −2.47 (0.19) −2.50 (0.22) −3.20 (0.41) MCI, mild cognitive impairment;
Log CSF p-tau181, pg/mL 2.80 (0.32) 2.91 (0.31) 3.32 (0.47) MMSE, Mini-Mental State
Examination; p-tau181, tau
Longitudinal biomarkers and cognition, phosphorylated at threonine 181;
yearly rates of change (SE)
PACC-3, Preclinical Alzheimer
Centiloids −0.17 (0.55) 0.0132 (0.6) 3.04 (1.98) Cognitive Composite;
Braak stages I/II FTP SUVR 0.01 (0.02) 0.02 (0.02) 0.06 (0.05) SUVR, standardized uptake
Braak stages III/IV FTP SUVR 0.01 (0.01) 0.02 (0.01) 0.07 (0.07) value ratio.
a
Only 915 participants had available
Braak stages V/VI FTP SUVR 0.01 (0.01) 0.01 (0.01) 0.04 (0.05)
APOE data (A− TMTL−, 241;
CU PACC-3b −0.06 (0.20) −0.09 (0.20) −0.14 (0.25) A− TMTL+, 245; A+ TMTL+, 429).
CI ADAS-Cog 11 1.30 (2.71) 1.61 (2.21) 3.11 (3.28) b
Sum of the z scores of the MMSE
Log CSF Aβ42/40 (1/y) −0.0027 (0.0005) −0.0027 (0.0007) −0.0034 (0.0006) total score, Log-Transformed Trail
Test B, and Logical Memory
Log CSF p-tau181, pg/mL/y 0.011 (0.013) 0.023 (0.022) 0.023 (0.017)
Delayed Recall.
To minimize the effect of subthreshold Aβ burden in the noassays. The CSF metrics used in this study included the
A− TMTL+ study group,30-32 Aβ positivity was defined using a baseline Aβ42/40 ratio (n = 359) and p-tau181 (n = 485) con-
conservative cutoff of 12 centiloids.27 This cut point proved to centrations, as well as follow-up measurements for a subset
optimally discriminate between Thal phases 0 to 1 and 2 to 533 of individuals (Aβ42/40: n = 77; mean [SD], 2.30 [1.03] years;
and it is therefore lower compared with traditional cut points p-tau181: n = 99; 2.34 [1.05] years).
based on discrimination of AD neuropathologic change levels
(24.4 centiloids33) or reliable worsening (19 centiloids34). The Cognitive Assessments
tau-positivity threshold was defined as the 95th percentile of Cognitive performance in cognitively unimpaired individu-
regional entorhinal cortex (ERC) SUVR values in the younger als was assessed using a modified version of the Preclinical Alz-
control cohort34 (SUVR = 1.21) (eFigure 1 in Supplement 1). heimer Cognitive Composite36 (PACC) derived as the sum of
the z scores of the Mini-Mental State Examination total score,
CSF Biomarkers Log-Transformed Trail Test B, and Logical Memory Delayed
Cerebrospinal fluid samples were collected for a subset of ADNI Recall (PACC-3). The PACC-3 is designed to detect the first
participants and processed according to previously de- signs of cognitive decline in otherwise asymptomatic indi-
scribed protocols.35 Concentrations of Aβ1-42, Aβ1-40, and tau viduals. Cognitive performance in cognitively impaired indi-
phosphorylated at threonine 181 (p-tau181) were measured by viduals (combined mild cognitive impairment and AD demen-
the ADNI Biomarker Core using the Roche Elecsys β-amyloid tia) was assessed using the Alzheimer’s Disease Assessment
(1-42), β-amyloid(1-40), and phospho-tau (181P) CSF immu- Scale–Cognitive Subscale (ADAS-Cog 11).
jamaneurology.com (Reprinted) JAMA Neurology Published online August 14, 2023 E3
Downloaded From: https://jamanetwork.com/ on 08/29/2023
Research Original Investigation Increased Medial Temporal Tau Uptake Without Amyloid-β Positivity
Statistical Analysis were found in baseline Braak stages I/II FTP-PET SUVR
Statistical analysis of differences between the A− TMTL− vs (d = 0.10; 95% CI, −0.02 to 0.23; P = .10) (eFigure 2 in Supple-
A− TMTL+ and A+ TMTL+ study groups was performed using ment 1), slightly higher longitudinal rates of Braak stages I/II
generalized linear models (GLMs) controlled for age, sex, co- FTP-PET SUVR change were observed in women (d = 0.13;
hort (ADNI, HABS, and AVID-A05), and baseline centiloid val- 95% CI, 0.02-0.23; P = .02). Both A− TMTL+ (mean [SD] age,
ues in the case of A− TMTL+ vs A− TMTL− comparisons. Effect 74.9 [7.6] years; d = 0.64; 95% CI, 0.47-0.83; P < .001) and
sizes were measured using Cohen d, and group differences A+ TMTL+ (age, 75.6 [8.0] years; d = 0.70; 95% CI, 0.55-0.86,
between cortical maps were corrected for multiple compari- P < .001) individuals were significantly older than the A− TMTL−
sons using the FreeSurfer clusterwise correction for multiple control cohort (age, 70.0 [7.8] years). Similarly, both the
comparisons. Longitudinal rates of change were computed A+ TMTL+ (60.4%; d = 0.69; 95% CI, 0.56-0.80; P < .001) and
using linear mixed-effect models with participant-specific A− TMTL+ (33.7%; d = 0.23; 95% CI, 0.06-0.40; P = .009)
intercepts and slopes (eg, Vk ~ time + (time|participant), where groups had a significantly higher proportion of cognitively
Vk is the value on the kth vertex of a cortical map). impaired individuals than the A− TMTL− group (24.4%). The
First, we investigated vertex-wise and ROI-based group dif- prevalence of apolipoprotein E (APOE)–ε4 was higher among
ferences in baseline FTP SUVRs. Vertex and ROI-based group A+ TMTL+ individuals (54.4%, d = 0.64; 95% CI, 0.54-0.76;
differences were also computed for the FTP SUVR longitudi- P < .001), but was similar between the A− TMTL+ (18.0%;
nal rates of change. Additionally, group differences in longi- d = −0.01; 95% CI, −0.19 to 0.16; P = .68) and the A− TMTL− con-
tudinal centiloid accumulation were similarly investigated. trol group (19.9%). By contrast, both the A− TMTL− (16.3%)
Analysis of baseline and longitudinal differences in CSF and A− TMTL+ groups (14.4%; d = 0.08; 95% CI, −0.10 to 0.25;
Aβ42/40 and p-tau181 biomarker levels used analogous sta- P = .38) showed significantly higher proportions of APOE-ε2
tistical models, but values were log-transformed before analy- carriers than the A+ TMTL+ group (4.4%; d = 0.38; 95% CI, 0.24-
sis to account for the exponential progression of CSF bio- 0.49; P < .001).
marker levels. Baseline and longitudinal differences across
groups in cognitive metrics were studied separately for cog- Tau and Aβ Accumulation
nitively unimpaired and cognitively impaired individuals Analysis of baseline FTP SUVR contrast maps (Figure 1A) noted
because of the different neuropsychological instruments that increased tau burden in A− TMTL+ individuals to be most pro-
are best suited to detect the subtle cognitive changes in par- nounced in the MTL and extending into the inferior temporal
ticipants without impairment and more overt cognitive changes lobe and the ventromedial prefrontal cortex, while A+ TMTL+
in those with impairment. As post hoc sensitivity analyses, we individuals showed the AD-characteristic pattern of wide-
repeated the previous analyses with higher cut points for Aβ spread cortical tau accumulation across temporal, parietal,
(24 centiloids) and tau PET positivity (mean +2.5 SD of the ERC and frontal areas. In vertex-wise longitudinal FTP SUVR analy-
FTP, SUVR = 1.27). Moreover, we assessed the outcome of ses, A− TMTL− individuals showed little increase of tau accu-
using a larger MTL ROI comprising the ERC and amygdala. mulation over time, whereas the A− TMTL+ cohort displayed
In addition to the comparisons of dichotomized A and a moderate increase of tau uptake restricted to the MTL and
TMTL groups, complementary analyses were performed to inferior temporal regions (Figure 1B). By contrast, A+ TMTL+
assess continuous associations of baseline ERC FTP SUVR with participants showed a pronounced and widespread increase
vertex-wise cortical thickness patterns across all A− individu- of tau accumulation. These differences were confirmed in di-
als, using GLMs adjusted by sex, age, cohort, and baseline cen- rect statistical contrasts between the TMTL+ groups and the
tiloid. Analogously, associations between baseline ERC FTP A− TMTL− group (Figure 1C). An ROI-based FTP SUVR analy-
SUVR and cognitive performance were studied across the sis showcased similar results (eFigure 3 in Supplement 1), with
A− subcohort with equally adjusted GLMs. Statistical tests were A− TMTL+ participants showing statistically significant albeit
2-sided, and P < .05 was considered statistically significant. moderate longitudinal (mean [SD], 1.83 [0.84] years) tau
The strength of the associations was assessed using the Pear- PET increases that were largely limited to the temporal lobe,
son partial correlation coefficient (r). whereas those with A+ TMTL+ showed faster and more corti-
cally widespread tau PET increases.
Regarding Aβ accumulation, centiloid rates of change in
A− TMTL+ participants did not show any significant increase
Results in centiloids over time (mean [SD], 0.01 [12]; P = .82) (eFig-
Demographic Characteristics ure 4 in Supplement 1), although the slopes were slightly dif-
Of the 965 individuals included in the study, 462 were men ferent from the slopes of the A− TMTL− group (−0.17 [0.55];
(47.9%) and 503 were women (52.1%); mean (SD) age was 73.9 d = 0.29; 95% CI, 0.04 to 0.54; P = .04) (Figure 1D). By con-
(8.1) years. A total of 51% A− individuals and 78% of A+ partici- trast, A+ TMTL+ individuals showed a pronounced increase in
pants had increased tau PET signal in the ERC (TMTL+) com- centiloids over time (3.04 [1.98] vs −0.17 [0.55]; d = 1.89;
pared with healthy younger (age, <39 years) controls. Further 95% CI, 1.64 to 2.24; P < .001).
demographic and biomarker characteristics are reported in the In the CSF subset analysis, A− TMTL+ participants showed
Table. Of participants with race data available (ie, ADNI and moderately higher baseline p-tau181 levels compared with the
HABS cohorts), 92.9% of the individuals were White. Al- A− TMTL− group (d = 0.24; 95% CI, 0.002-0.48; P = .04), but
though no significant differences between women and men no significant difference in Aβ42/40 (d = −0.10; 95% CI, −0.34
E4 JAMA Neurology Published online August 14, 2023 (Reprinted) jamaneurology.com
Downloaded From: https://jamanetwork.com/ on 08/29/2023
Increased Medial Temporal Tau Uptake Without Amyloid-β Positivity Original Investigation Research
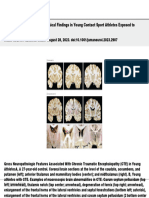
Figure 1. Cross-Sectional and Longitudinal Characterization of Amyloid-β (A) and Tau (T) Positron Emission Tomography Accumulation
in the Medial Temporal Lobe (MTL)
A Group differences in cross-sectional FTP SUVR patterns
A− TMTL− < A− TMTL+ A− TMTL− < A+ TMTL+ A− TMTL+ < A+ TMTL+
d d
0.6 1 0.4 0.8
B Average longitudinal FTP SUVR patterns across groups
A− TMTL− A− TMTL+ A+ TMTL+
SUVR/y
0.02 0.004
C Group differences in longitudinal FTP SUVR progression patterns D Longitudinal centiloid change
A− TMTL− < A− TMTL+ A− TMTL− < A+ TMTL+ A− TMTL+ < A+ TMTL+ Centiloid change rate, centiloid/y 10 P <.001
4 P = .04
d d 0
0.6 1 0.4 0.8
–2
A− TMTL− A− TMTL+ A+ TMTL+
A, Vertex-wise group differences in cross-sectional [18F]flortaucipir (FTP) standardized uptake value ratio (SUVR) patterns in A− TMTL+ and A+ TMTL+ individuals
compared with the control group, expressed as Cohen d. B, Average longitudinal FTP SUVR patterns in A− TMTL−, A− TMTL+, and A+ TMTL+ individuals,
represented as vertex-wise rates of changes. C, Vertex-wise group differences in longitudinal FTP SUVR in A− TMTL+ and A+ TMTL+ individuals compared with
the A− TMTL− control group, expressed as Cohen d. D, Longitudinal centiloid change in A− TMTL−, A− TMTL+, and A+ TMTL+ individuals.
to 0.14; P = .38) (Figure 2A), whereas A+ TMTL+ individuals ex- Neurodegeneration
hibited the expected alterations in both Aβ42/40 (d = −1.38; Compared with the A− TMTL− group, A− TMTL+ participants
95% CI, −1.66 to −1.14; P < .001) and p-tau181 (d = 1.00; (Figure 3A) showed cortical thinning at baseline mainly re-
95% CI, 0.81-1.20; P < .001) levels (Figure 2A). In longitudinal stricted to the MTL, whereas A+ TMTL+ participants showed
analyses, the A− TMTL+ group showed larger increases in more widespread cortical thinning extending to the lateral tem-
p-tau181 levels over time at trend-level statistical signifi- poral lobe, the posterior cingulate, and the parietal and fron-
cance (d = 0.52; 95% CI, 0.08-1.04; P = .07), but no signifi- tal lobes. This pattern was also reflected in ROI-based analy-
cant difference in Aβ42/40 ratio change (d = 0.34; 95% CI, −0.16 ses, with A− TMTL+ individuals showing significant cortical
to 0.94; P = .22), compared with the A − TMTL − group thinning in Braak stages I/II and Braak stages III/IV only
(Figure 2B). The A+ TMTL+ group showed significantly faster (Figure 3B). The complementary analysis using continuous
rates of change in both biomarkers (Aβ42/40: d = −1.33; tau PET measures confirmed an association between ERC FTP
95% CI, −1.92 to −0.87; P < .001; p-tau181: d = 0.53; 95% CI, SUVR and medial temporal neurodegeneration across A−
0.07-1.06; P = .04). individuals (eFigure 5 in Supplement 1).
jamaneurology.com (Reprinted) JAMA Neurology Published online August 14, 2023 E5
Downloaded From: https://jamanetwork.com/ on 08/29/2023
Research Original Investigation Increased Medial Temporal Tau Uptake Without Amyloid-β Positivity
Figure 2. Baseline and Longitudinal Characterization of Cerebrospinal Fluid (CSF)
Amyloid-β (Aβ) and Tau Biomarkers
A CSF biomarker distribution at baseline
–1.5 P <.001 5.0
P <.001
Log-transformed CSF Aβ42/Aβ40
Log-transformed CSF p-tau181,
P = .38 4.5
–2.0
4.0
P = .04
–2.5
pg/mL
3.5
–3.0
3.0
–3.5
2.5
–4.0 2.0
A− TMTL− A− TMTL+ A+ TMTL+ A− TMTL− A− TMTL+ A+ TMTL+
(n = 76) (n = 99) (n = 184) (n = 100) (n = 134) (n = 251)
B Longitudinal CSF biomarker change
P = .04
0 0.12
P <.001
P = .07
Log-transformed CSF Aβ42/Aβ40
Log-transformed CSF p-tau181
–0.001 0.10
P = .22
change rate, pg/mL/y
0.08
change rate, 1/y
–0.002
0.06
–0.003 A, Baseline levels of CSF Aβ42/40
0.04 and tau phosphorylated at threonine
–0.004 181 (p-tau181) across groups.
0.02
Biomarker levels were statistically
–0.005
0 compared using generalized linear
–0.006 models (GLMs). B, Longitudinal
–0.02
change of CSF Aβ42/40 and p-tau181
A− TMTL− A− TMTL+ A+ TMTL+ A− TMTL− A− TMTL+ A+ TMTL+ metrics across groups, obtained using
(n = 19) (n = 31) (n = 27) (n = 25) (n = 27) (n = 47) linear mixed-effect models and
statistically compared with GLMs.
In longitudinal analyses, A− TMTL+ individuals showed significantly faster cognitive deterioration (d = 0.60; 95% CI,
faster cortical thinning compared with the A− TMTL− group that 0.30-0.90; P < .001) (eFigure 6 in Supplement 1). Among
was largely restricted to the MTL, while accelerated cortical cognitively unimpaired participants, neither A − TMTL +
thinning in the A+ TMTL+ group further extended to the lat- (d = −0.06; 95% CI, −0.25 to 0.40; P = .61) nor A+ TMTL+
eral temporal, parietal, and frontal lobes (Figure 3C). Simi- (d = −0.17; 95% CI, −0.44 to 0.14; P = .15) individuals showed
larly, ROI-wise analyses (Figure 3D) showed significantly faster a significant difference in PACC-3 decline compared with
cortical thinning in A− TMTL+ participants, mostly in Braak the A− TMTL− group. In the complementary analysis with
stages I/II. continuous tau PET measures, baseline ERC FTP SUVR
was significantly correlated with faster cognitive decline in
Cognition cognitively impaired A− individuals (ADAS-Cog 11: r = 0.30;
At baseline, cognitively impaired A + TMTL + individuals 95% CI, 0.15-0.53; P < .001), but not in cognitively unim-
showed lower ADAS-Cog 11 scores compared with the cogni- paired A− individuals (PACC-3: r = 0.03; 95% CI, −0.22 to
tively impaired A− TMTL− group (d = −0.57; 95% CI, −0.79 to 0.14; P = .63).
−0.34; P < .001), but neither the cognitively impaired A− TMTL+
individuals nor any of the cognitively unimpaired groups Sensitivity Analyses
differed significantly from the respective A− TMTL− con- Overall, the results derived from the sensitivity analy-
trols (Figure 4). In the complementary analysis with continu- ses were consistent with the main results presented
ous tau PET measures, baseline ERC FTP SUVR was sig- in this study. Similar patterns of tau PET SUVR, CSF bio-
nificantly correlated with worse cognition in cognitively markers, atrophy, and clinical change were found across
impaired A − individuals (ADAS-Cog 11: r = 0.26; 95% CI, the A TMTL groups when changing the Aβ PET cut point to
0.08-0.46; P = .001), but not in cognitively unimpaired 2 4 c e nt i l o i d s (e F i g u re s 7-1 0 i n S u p p l e m e nt 1 ) a n d
A − individuals (PACC-3: r = 0.02; 95% CI, −0.09 to 0.13; when changing the Braak stages I/II SUVR cut point to
P = .72). 1.27 (eFigures 11-14 in the Supplement 1). Analyses using
Longitudinally, cognitively impaired individuals with A− a larger MTL ROI (ERC plus amygdala) yielded a slight-
TMTL+ showed a comparable degree of moderate decline in ly different distribution of A TMTL groups (eFigure 15 in
ADAS-Cog 11 scores compared with cognitively impaired A− Supplement 1) and showed that the Aβ- and tau- accumula-
TMTL− individuals (d = 0.25; 95% CI, −0.11 to 0.58; P = .18), tion patterns were similar to those obtained with the ERC
whereas cognitively impaired A+ TMTL+ participants showed ROI.
E6 JAMA Neurology Published online August 14, 2023 (Reprinted) jamaneurology.com
Downloaded From: https://jamanetwork.com/ on 08/29/2023
Figure 3. Baseline and Longitudinal Characterization of Atrophy, as Shown With Structural Magnetic Resonance Imaging
A Vertex-wise group differences in baseline cortical thickness B ROI-wise cortical thickness at baseline
A− TMTL− < A− TMTL+ A− TMTL− < A+ TMTL+ A− TMTL+ < A+ TMTL+ 5 P <.001 3.50
P <.001
jamaneurology.com
P <.001 3.25
4 P = .03
P = .35
3.00
2.75 P = .36
3
2.50
2
2.25
d
Average cortical thickness, mm
Average cortical thickness, mm
–0.20 –0.50 2.00
1
Downloaded From: https://jamanetwork.com/ on 08/29/2023
A− TMTL− A− TMTL+ A+ TMTL+ A− TMTL− A− TMTL+ A+ TMTL+ A− TMTL− A− TMTL+ A+ TMTL+
Braak I/II Braak III/IV Braak V/VI
C Vertex-wise group differences in longitudinal cortical thickness change D ROI-wise longitudinal cortical thickness change
Increased Medial Temporal Tau Uptake Without Amyloid-β Positivity
A− TMTL− < A− TMTL+ A− TMTL− < A+ TMTL+ A− TMTL+ < A+ TMTL+ P <.001
0.075 P <.001 0.015
P <.001 P <.001
0.050
P = .04 0.010
0.025 P = .04
0.005
0
–0.025 0
–0.050
–0.005
–0.075
d –0.010
–0.20 –0.50 –0.100
–0.125 –0.015
Cortical thickness change rate, mm/y
Cortical thickness change rate, mm/y
A− TMTL− A− TMTL+ A+ TMTL+ A− TMTL− A− TMTL+ A+ TMTL+ A− TMTL− A− TMTL+ A+ TMTL+
Braak I/II Braak III/IV Braak V/VI
A, Vertex-wise group differences in baseline cortical thickness patterns in A− TMTL+ and A+ TMTL+ individuals compared with the A− TMTL− control group, measured as Cohen d. B, Regional (region of interest [ROI]) group differences
in baseline cortical thickness patterns in A− TMTL+ (Braak stages I/II: d = −0.50; 95% CI, −0.65 to −0.34; P < .001; Braak stages III/IV: d = −0.20; 95% CI, −0.38 to −0.02; P = .025; Braak stages V/VI: d = −0.08; 95% CI, −0.26 to 0.10;
P = .36), and A+ TMTL+ individuals (Braak stages I/II: d = −0.60; 95% CI, −0.73 to −0.46; P < .001; Braak stages III/IV: d = −0.34; 95% CI, −0.49 to −0.20; P < .001; Braak stages V/VI: d = −0.17; 95% CI, −0.32 to −0.02; P = .035)
compared with the A− TMTL− control group using generalized linear models (GLM). C, Vertex-wise group differences in longitudinal cortical thickness progression patterns in A− TMTL+, and A+ TMTL+ individuals compared with
the A− TMTL− control group, measured as Cohen d. D, Regional group differences in longitudinal cortical thickness progression patterns in A− TMTL+ (Braak stages I/II: d = −0.38; 95% CI, −0.60 to −0.16; P < .001; Braak stages III/IV:
d = −0.22; 95% CI, −0.44 to 0.008; P = .044; Braak stages V/VI: d = −0.22; 95% CI, −0.44 to −0.005; P = .041), and A+ TMTL+ individuals (Braak stages I/II: d = −0.76; 95% CI, −0.95 to −0.59; P < .001; Braak stages III/IV: d = −0.57;
95% CI, −0.76 to −0.39; P < .001; Braak stages V/VI: d = −0.34; 95% CI, −0.53 to −0.16; P < .001) compared with the A− TMTL− control group using GLM models.
Original Investigation Research
(Reprinted) JAMA Neurology Published online August 14, 2023
E7
Research Original Investigation Increased Medial Temporal Tau Uptake Without Amyloid-β Positivity
Figure 4. Baseline and Longitudinal Characterization of Cognitive Performance
A Cognitive performance at baseline
P <.001 P = .42
50 7.5
P = .19 P = .38
5.0
40
2.5
CI ADAS-Cog 11
CU PACC-3
30 0
20 –2.5
–5.0
10
–7.5
A, Baseline Alzheimer’s Disease
0 –10.0
Assessment Scale–Cognitive Subscale
A− TMTL− A− TMTL+ A+ TMTL+ A− TMTL− A− TMTL+ A+ TMTL+ (ADAS-Cog 11) scores in cognitively
impaired (CI) individuals and sum of
the z scores of the Mini-Mental
B Longitudinal cognitive performance change
P = .15 State Examination total score,
17.5 P <.001 0.75 Log-Transformed Trail Test B,
CI ADAS-Cog 11 change rate, 1/y
P = .61 and Logical Memory Delayed Recall
15.0 P = .18 0.50
CU PACC-3 change rate, 1/y
scores (PACC-3) in cognitively
12.5 0.25 unimpaired (CU) individuals across
10.0 amyloid-β (A) and tau (T) medial
0
temporal lobe (MTL) groups,
7.5 –0.25 compared using generalized linear
5.0 models (GLMs). B, Longitudinal
–0.50
changes in ADAS-Cog 11 scores in
2.5
–0.75 CI individuals and PACC-3 scores in
0 CU individuals across A and TMTL
–1.00
groups, obtained with linear
A− TMTL− A− TMTL+ A+ TMTL+ A− TMTL− A− TMTL+ A+ TMTL+ mixed-effect models and compared
using GLMs.
local tau pathology needs to reach a certain density of neuro-
Discussion fibrillary tangles to be detected in an FTP-PET scan, which is
mostly the case for Braak stages V/VI.38-41 This may lead to the
In this study, we explored in detail the pathologic and clinical conclusion that FTP-PET cannot detect PART-related tau
course of older individuals who display PET-measured tau ac- deposition, which is, by definition, Braak stage IV or less. Yet,
cumulation in the MTL in the absence of Aβ pathology (A− FTP showed binding to neurofibrillary tangles from PART
TMTL+), a condition reminiscent of pathologically defined brains in autoradiography studies42,43 and, therefore, FTP-
PART.5 In a large multicentric cohort of almost 1000 older in- PET may detect a subset of PART cases with suprathreshold
dividuals, we found that increased MTL tau PET signal with- neurofibrillary tangle density. To date, the number of PART
out notable Aβ pathology is relatively common in older indi- cases in the available PET-to-autopsy studies is low (n = 3)38
viduals and is associated with further longitudinal tau PET and we cannot exclude that PART could be detected with FTP-
uptake increase, which remains largely restricted to the PET in a subset of individuals. This hypothesis is consistent
MTL. These tau PET increases colocalize with progressive with the fact that the prevalence of tau PET positivity among
MTL neurodegeneration, are associated with only subtle older A− individuals in our study (51%) is considerably lower
changes in global cognitive performance, and are not accom- than the prevalence of PART in this age range in neuropatho-
panied by notable accumulation of Aβ pathology over time. logic studies.5 The topography of our findings is also consis-
Using a tau PET cutoff defined in healthy younger indi- tent with PART: in line with recent studies,15,44,45 our results
viduals, we observed that tau PET-measured MTL accumula- showed that increased tau PET signal in A− TMTL+ individu-
tion in the absence of Aβ is a common condition in older in- als was largely limited to the MTL. Both baseline and longitu-
dividuals, representing 51% of A− individuals in this study. dinal increases in tau PET signal in A− TMTL+ individuals were
The frequency of tau PET positivity in this A− sample is con- found to be paralleled by increases in CSF p-tau181 levels, sug-
sistent with a previous study using a similar method (67%),17 gesting that these signal increases reflect actual increases in
and it is substantially higher compared with previous studies tau burden. Together, these results suggest that PART may be
using larger temporal ROIs without partial volume correction an important neuropathologic substrate for many A− TMTL+
(approximately 17%-20%).15,37 The discrepancy may be ex- individuals in our study, although probably not the only one.15
plained by use of extra-MTL ROIs without partial volume We also acknowledge that pathologic entities other than
correction, which results in a lack of sensitivity to MTL- PART may lead to abnormal FTP-PET signal in the MTL among
specific signal. Aβ-negative individuals. Although FTP shows high spe-
The degree to which FTP-PET can detect PART remains a cificity for AD-type tau aggregates in autoradiography
subject of debate. PET-to-autopsy studies generally agree that studies,42,46,47 extensive increases in cortical FTP-PET signal
E8 JAMA Neurology Published online August 14, 2023 (Reprinted) jamaneurology.com
Downloaded From: https://jamanetwork.com/ on 08/29/2023
Increased Medial Temporal Tau Uptake Without Amyloid-β Positivity Original Investigation Research
in the absence of Aβ can occur in patients with AD dementia association of ERC tau uptake with worse cognition and faster
or mild cognitive impairment, which likely represent tangle- cognitive decline also in cognitively impaired A− partici-
predominant dementia.15,48 Moreover, FTP-PET increases can pants. These results suggest that, in the absence of Aβ, tau ac-
occur in frontotemporal dementia syndromes, including cumulation in the MTL has only subtle effects on cognition
those associated with tau and TAR DNA-binding protein 43 and does not herald the pronounced cognitive decline typical
(TDP-43).49-52 The binding mechanisms remain unclear, al- for AD. Further research with longer follow-up might be nec-
though binding to non-AD tau as well as to neurodegenera- essary to delineate the long-term consequences of ongoing tau
tive processes that colocalize with TDP-43 deposition might accumulation in the absence of Aβ.
result in nonspecific FTP binding.49,53 Therefore, we cannot
exclude the possibility that limbic-predominant age-related Limitations
TDP-43 encephalopathy, which is associated with neurode- This study has limitations. The first of these is the lack of au-
generation in the MTL, might also result in abnormal FTP- topsy data of A− TMTL+ individuals, which leaves the exact as-
PET signal in the same regions, although the influence of sociation between the PET-defined A− TMTL+ group and PART
limbic-predominant age-related TDP-43 encephalopathy on to be determined. Second, we relied on cutoffs for group defi-
FTP-PET appears to be limited.54 These considerations sug- nition. While centiloid cutoffs for denoting Aβ status are well
gest that the A− TMTL+ group likely represents both a clini- established,27 a number of different methods and cutoffs for
cally and pathologically heterogeneous group. Thus, in- defining tau PET positivity have been used in the literature,
creased FTP-PET signal in the MTL in the absence of Aβ should resulting in highly variable proportions of the different A and
not be considered a specific marker of PART. Despite these TMTL groups.57 Herein, we applied a commonly used method
limitations, our findings are valuable and contribute to un- for objectively defining biomarker cutoffs based on data from
derstanding the clinical and pathologic course of the A− TMTL+ healthy younger controls,34 and several of our principal find-
group as a whole. Yet, given its high frequency among cogni- ings were replicated in complementary continuous analyses
tively unimpaired individuals and those with mild average that are independent of cutoff definition. Third, to achieve ro-
cognitive decline, the clinical significance of the A− TMTL+ pro- bust sample sizes of the less-prevalent A− TMTL+ individuals,
file remains uncertain. Additional work is needed to identify we pooled data across different cohorts. While the possible in-
the subset of A− TMTL+ individuals who will experience more fluence of multicentric data acquisitions was minimized by
relevant clinical outcomes. harmonizing imaging preprocessing, it limited our ability
Given that longitudinal follow-up of neuropathologically to analyze domain-specific cognitive decline, as neuro-
defined PART is not possible, a main controversy exists whether psychological instruments differed across cohorts. Fourth,
PART reflects an early form of AD, with Aβ pathology devel- follow-up time for the evaluation of both longitudinal clini-
oping in the further disease course, or whether it represents cal and biomarker measures was relatively short. Fifth, the co-
an entirely distinct pathologic entity.12-14 Herein, we noted that horts included in our study represent selective research co-
A− TMTL+ individuals did not show significant Aβ accumula- horts that may not reflect the general population, and our
tion over the available follow-up period, thus arguing against findings should be replicated in more diverse cohorts.
the possibility that this condition reflects an early tau-first
subtype of AD.55
Longitudinal cortical thickness analysis demonstrated that
A− TMTL+ individuals have moderate and restricted MTL at-
Conclusions
rophy progression, whereas atrophy is more accelerated and The results presented in this longitudinal cohort study sug-
spreads to widespread neocortical regions in A+ TMTL+ par- gest that individuals with MTL tau accumulation in the ab-
ticipants. These results suggest that tau accumulation in Aβ- sence of Aβ follow a separate, less malign, pathologic course
negative individuals is not a benign process but is associated compared with that of typical AD. While these individuals
with increased neurodegeneration,56 although the rates of pro- showed progressive tau accumulation and neurodegenera-
gression are significantly slower compared with rates in A+ tion, this process was comparably slow, remained largely re-
TMTL+ participants. stricted to the MTL, and was associated with only subtle
In cognition analyses, we did not find significant differ- changes in global cognitive performance. Moreover, these in-
ences in baseline performance or longitudinal decline be- dividuals did not show notable Aβ accumulation over follow-
tween A− TMTL+ individuals and the A− TMTL− controls, up, arguing against the possibility that this A− TMTL+ condi-
whereas a significantly faster decline was observed in cogni- tion reflects a tau-first subtype of AD. Further studies are
tively impaired A+ TMTL+ individuals. However, a more sen- warranted that specify the exact association of this common
sitive analysis using continuous tau measures showed an PET-defined condition with pathologic PART.
ARTICLE INFORMATION Open Access: This is an open access article Department and Molecular Imaging Group,
Accepted for Publication: May 16, 2023. distributed under the terms of the CC-BY License. Instituto de Investigación Sanitaria de Santiago de
© 2023 Costoya-Sánchez A et al. JAMA Neurology. Compostel, Travesía da Choupana s/n, Santiago de
Published Online: August 14, 2023. Compostela, Spain (Costoya-Sánchez, Aguiar);
doi:10.1001/jamaneurol.2023.2560 Author Affiliations: Universidade de Santiago de
Compostela, Santiago de Compostela, Spain Centro de Investigación Biomédica en Red sobre
(Costoya-Sánchez, Aguiar); Nuclear Medicine Enfermedades Neurodegenerativas, Instituto de
jamaneurology.com (Reprinted) JAMA Neurology Published online August 14, 2023 E9
Downloaded From: https://jamanetwork.com/ on 08/29/2023
Research Original Investigation Increased Medial Temporal Tau Uptake Without Amyloid-β Positivity
Salud Carlos III, Madrid, Spain (Costoya-Sánchez, the Swedish Research Council (2017-02869, Hospital/Harvard Medical School, Boston.
Silva-Rodríguez, Aguiar, Grothe); Wallenberg Centre 2021-02678 and 2021-06545), the European Dr Devous retired.
for Molecular and Translational Medicine, University Union’s Horizon Europe research and innovation
of Gothenburg, Gothenburg, Sweden (Moscoso, program under grant agreement no 101132933 REFERENCES
Schöll, Grothe); Department of Psychiatry and (AD-RIDDLE), the National Institute of Health 1. Duyckaerts C, Delatour B, Potier M-C.
Neurochemistry, Institute of Physiology and (R01 AG081394-01), the Swedish state under the Classification and basic pathology of Alzheimer
Neuroscience, University of Gothenburg, agreement between the Swedish government and disease. Acta Neuropathol. 2009;118(1):5-36.
Gothenburg, Sweden (Moscoso, Schöll); Unidad de the County Councils, the ALF-agreement doi:10.1007/s00401-009-0532-1
Trastornos del Movimiento, Servicio de Neurología (ALFGBG-813971 and ALFGBG-965326), 2. Serrano-Pozo A, Frosch MP, Masliah E,
y Neurofisiología Clínica, Instituto de Biomedicina the Swedish Brain Foundation (FO2021-0311) and Hyman BT. Neuropathological alterations in
de Sevilla, Hospital Universitario Virgen del Rocío/ the Swedish Alzheimer Foundation (AF-740191). Alzheimer disease. Cold Spring Harb Perspect Med.
CSIC/Universidad de Sevilla, Seville, Spain Data collection and sharing for this project was 2011;1(1):a006189-a006189. doi:10.1101/cshperspect.
(Silva-Rodríguez, Grothe); Avid funded by the Alzheimer’s Disease Neuroimaging a006189
Radiopharmaceuticals, Philadelphia, Pennsylvania Initiative (ADNI) (National Institutes of Health grant
3. Bennett DA, Schneider JA, Wilson RS, Bienias JL,
(Pontecorvo, Devous); Eli Lilly and Company, U01 AG024904) and Department of Defense ADNI Arnold SE. Neurofibrillary tangles mediate the
Indianapolis, Indiana (Pontecorvo, Devous); (award W81XWH-12-2-0012). The ADNI is funded association of amyloid load with clinical Alzheimer
Dementia Research Centre, Institute of Neurology, by the National Institute on Aging, and the National disease and level of cognitive function. Arch Neurol.
University College London, London, United Institute of Biomedical Imaging and Bioengineering, 2004;61(3):378-384. doi:10.1001/archneur.61.3.378
Kingdom (Schöll). and through generous contributions from the
4. Braak H, Thal DR, Ghebremedhin E,
Author Contributions: Mr Costoya-Sánchez and following: AbbVie, Alzheimer’s Association,
Del Tredici K. Stages of the pathologic process in
Dr Moscoso contributed equally to the study, had Alzheimer’s Drug Discovery Foundation, Araclon Alzheimer disease: age categories from 1 to 100
full access to all of the data in the study, and take Biotech, BioClinica Inc, Biogen, Bristol-Myers years. J Neuropathol Exp Neurol. 2011;70(11):
responsibility for the integrity of the data and the Squibb Company, CereSpir Inc, Cogstate, Eisai Inc, 960-969. doi:10.1097/NEN.0b013e318232a379
accuracy of the data analysis. Equally contributing Elan Pharmaceuticals Inc, Eli Lilly and Company,
5. Crary JF, Trojanowski JQ, Schneider JA, et al.
last authors: Drs Aguiar, Schöll, and Grothe. EuroImmun, F. Hoffmann-La Roche Ltd and its
Primary age-related tauopathy (PART): a common
Concept and design: Costoya-Sánchez, Moscoso, affiliated company Genentech Inc, Fujirebio, GE
pathology associated with human aging. Acta
Devous, Schöll, Grothe. Healthcare, IXICO Ltd, Janssen Alzheimer Neuropathol. 2014;128(6):755-766. doi:10.1007/
Acquisition, analysis, or interpretation of data: Immunotherapy Research & Development LLC, s00401-014-1349-0
All authors. Johnson & Johnson Pharmaceutical Research &
Development LLC, Lumosity, Lundbeck, Merck & Co 6. Josephs KA, Murray ME, Tosakulwong N, et al.
Drafting of the manuscript: Costoya-Sánchez, Tau aggregation influences cognition and
Moscoso, Aguiar, Grothe. Inc, Meso Scale Diagnostics LLC, NeuroRx Research,
hippocampal atrophy in the absence of
Statistical analysis: Costoya-Sánchez, Moscoso, Neurotrack Technologies, Novartis Pharmaceuticals
beta-amyloid: a clinico-imaging-pathological study
Grothe. Corp, Pfizer Inc, Piramal Imaging, Servier,
of primary age-related tauopathy (PART). Acta
Obtained funding: Aguiar, Schöll. Takeda Pharmaceutical Company, and Transition Neuropathol. 2017;133(5):705-715. doi:10.1007/
Administrative, technical, or material support: Therapeutics. The Canadian Institutes of Health s00401-017-1681-2
Silva-Rodríguez, Aguiar. Research is providing funds to support ADNI clinical
7. Besser LM, Crary JF, Mock C, Kukull WA.
Supervision: Moscoso, Silva-Rodríguez, Devous, sites in Canada. Private sector contributions are
Comparison of symptomatic and asymptomatic
Aguiar, Schöll, Grothe. facilitated by the Foundation for the National
persons with primary age-related tauopathy.
Institutes of Health (www.fnih.org). The grantee
Conflict of Interest Disclosures: Neurology. 2017;89(16):1707-1715. doi:10.1212/WNL.
organization is the Northern California Institute for 0000000000004521
Dr Silva-Rodríguez reported that he is a founder Research and Education, and the study is
and advisor for Qubiotech Health Intelligence SL, coordinated by the Alzheimer’s Therapeutic 8. Jefferson-George KS, Wolk DA, Lee EB,
a company commercializing neuroimaging McMillan CT. Cognitive decline associated with
Research Institute at the University of Southern
quantification software. Dr Pontecorvo reported pathological burden in primary age-related
California.
being an Eli Lilly and Company employee and minor tauopathy. Alzheimers Dement. 2017;13(9):
stockholder outside the submitted work. Dr Devous Role of the Funder/Sponsor: The funding 1048-1053. doi:10.1016/j.jalz.2017.01.028
reported being an Eli Lilly and Company employee organizations had no role in the design and conduct 9. Bell WR, An Y, Kageyama Y, et al.
and minor stockholder outside the submitted work. of the study; collection, management, analysis, and Neuropathologic, genetic, and longitudinal
Dr Aguiar reported being a cofounder of Qubiotech interpretation of the data; preparation, review, or cognitive profiles in primary age-related tauopathy
Health Intelligence SL. Dr Schöll has served on approval of the manuscript; and decision to submit (PART) and Alzheimer’s disease. Alzheimers Dement.
advisory boards for Roche and Novo Nordisk the manuscript for publication. 2019;15(1):8-16. doi:10.1016/j.jalz.2018.07.215
(outside scope of submitted work). No other Group Information: Members of the Alzheimer’s 10. Besser LM, Mock C, Teylan MA, Hassenstab J,
disclosures were reported. Disease Neuroimaging Initiative and the Harvard Kukull WA, Crary JF. Differences in cognitive
Funding/Support: We thank AVID Aging Brain Study appear in Supplement 2. impairment in primary age-related tauopathy
versus Alzheimer disease. J Neuropathol Exp Neurol.
Radiopharmaceuticals for supporting this Data Sharing Statement: See Supplement 3.
2019;78(3):219-228. doi:10.1093/jnen/nly132
study by facilitating access to the AVID-A05 data. Additional Information: Part of the data used in
The project that gave rise to these results received 11. Jack CR Jr, Bennett DA, Blennow K, et al;
preparation of this article were obtained from the
the support of a fellowship from la Caixa Contributors. NIA-AA Research Framework: toward
Alzheimer’s Disease Neuroimaging Initiative (ADNI) a biological definition of Alzheimer’s disease.
Foundation (ID 100010434). The fellowship database (adni.loni.usc.edu). As such, the
code is LCF/BQ/DR20/11790012. Dr Aguiar and Alzheimers Dement. 2018;14(4):535-562. doi:10.
investigators within the ADNI contributed to the 1016/j.jalz.2018.02.018
Mr Costoya-Sánchez are supported by the research design and implementation of ADNI and/or
grant PI9/01315 of the Instituto de Salud Carlos 12. Duyckaerts C, Braak H, Brion JP, et al. PART is
provided data but did not participate in the analysis
III-Fondo Europeo de Desarrollo Regional part of Alzheimer disease. Acta Neuropathol. 2015;
or the writing of this report. A complete listing of
(ISCIII-FEDER) and by the Centro de Investigaciones 129(5):749-756. doi:10.1007/s00401-015-1390-7
ADNI investigators can be found at https://adni.loni.
Biomédicas en Red (CIBER, CB22/05/00067). usc.edu/wp-content/uploads/how_to_apply/ADNI_ 13. Jellinger KA, Alafuzoff I, Attems J, et al. PART,
Dr Grothe is supported by the Miguel Servet Acknowledgement_List.pdf.pdf . Part of the data a distinct tauopathy, different from classical
program (CP19/00031) and research grant PI20/ sporadic Alzheimer disease. Acta Neuropathol.
used in the preparation of this article were obtained
00613 of the ISCIII-FEDER. Dr. Silva-Rodríguez is 2015;129(5):757-762. doi:10.1007/s00401-015-1407-2
from the Harvard Aging Brain Study
supported by the “Sara Borrell” program (CD21/ (HABS-P01AG036694; https://habs.mgh.harvard. 14. Jack CR Jr. PART and SNAP. Acta Neuropathol.
00067) of the ISCIII-FEDER. Dr Schöll is supported edu). The HABS study was launched in 2010, 2014;128(6):773-776.
by the Knut and Alice Wallenberg Foundation funded by the National Institute on Aging, and is led doi:10.1007/s00401-014-1362-3
(Wallenberg Centre for Molecular and Translational by principal investigators Reisa A. Sperling, MD, and 15. Yoon B, Guo T, Provost K, et al; Alzheimer’s
Medicine; KAW2014.0363 and KAW 2023.0371), Keith A. Johnson, MD, at Massachusetts General Disease Neuroimaging Initiative. Abnormal tau in
E10 JAMA Neurology Published online August 14, 2023 (Reprinted) jamaneurology.com
Downloaded From: https://jamanetwork.com/ on 08/29/2023
Increased Medial Temporal Tau Uptake Without Amyloid-β Positivity Original Investigation Research
amyloid PET negative individuals. Neurobiol Aging. surface-based coordinate system. Neuroimage. correlates with postmortem neurofibrillary tangle
2022;109:125-134. doi:10.1016/j.neurobiolaging.2021. 1999;9(2):195-207. doi:10.1006/nimg.1998.0396 Braak staging. Acta Neuropathol. 2017;134(4):
09.019 30. Diedrichsen J. A spatially unbiased atlas 619-628. doi:10.1007/s00401-017-1740-8
16. Altomare D, Caprioglio C, Assal F, et al. template of the human cerebellum. Neuroimage. 44. Groot C, Doré V, Robertson J, et al. Mesial
Diagnostic value of amyloid-PET and tau-PET: 2006;33(1):127-138. doi:10.1016/j.neuroimage.2006. temporal tau is related to worse cognitive
a head-to-head comparison. Eur J Nucl Med Mol 05.056 performance and greater neocortical tau load in
Imaging. 2021;48(7):2200-2211. doi:10.1007/s00259- 31. Mormino EC, Insel PS. Uncertainties in the PET amyloid-β–negative cognitively normal individuals.
021-05246-x defined A-/TNeocortical+ subtype. Alzheimers Neurobiol Aging. 2021;97:41-48. doi:10.1016/j.
17. Weigand AJ, Bangen KJ, Thomas KR, et al; Dement (Amst). 2022;14(1):e12348. neurobiolaging.2020.09.017
Alzheimer’s Disease Neuroimaging Initiative. Is tau 32. Krishnadas N, Doré V, Laws SM, et al. Exploring 45. Krishnadas N, Doré V, Groot C, et al;
in the absence of amyloid on the Alzheimer’s discordant low amyloid beta and high neocortical AIBL research group. Mesial temporal tau in
continuum? a study of discordant PET positivity. tau positron emission tomography cases. amyloid-β–negative cognitively normal older
Brain Commun. 2020;2(1):fcz046. doi:10.1093/ Alzheimers Dement (Amst). 2022;14(1):e12326. persons. Alzheimers Res Ther. 2022;14(1):51.
braincomms/fcz046 doi:10.1002/dad2.12326 doi:10.1186/s13195-022-00993-x
18. Jagust WJ, Landau SM, Shaw LM, et al; 33. La Joie R, Ayakta N, Seeley WW, et al. Multisite 46. Marquié M, Normandin MD, Vanderburg CR,
Alzheimer’s Disease Neuroimaging Initiative. study of the relationships between antemortem et al. Validating novel tau positron emission
Relationships between biomarkers in aging and [11C]PIB-PET centiloid values and postmortem tomography tracer [F-18]-AV-1451 (T807) on
dementia. Neurology. 2009;73(15):1193-1199. measures of Alzheimer’s disease neuropathology. postmortem brain tissue. Ann Neurol. 2015;78(5):
doi:10.1212/WNL.0b013e3181bc010c Alzheimers Dement. 2019;15(2):205-216. doi:10. 787-800. doi:10.1002/ana.24517
19. Dagley A, LaPoint M, Huijbers W, et al. 1016/j.jalz.2018.09.001 47. Marquié M, Normandin MD, Meltzer AC, et al.
Harvard Aging Brain Study: dataset and 34. Jack CR Jr, Wiste HJ, Weigand SD, et al. Pathological correlations of [F-18]-AV-1451 imaging
accessibility. Neuroimage. 2017;144(pt B):255-258. Defining imaging biomarker cut points for brain in non-Alzheimer tauopathies. Ann Neurol. 2017;81
doi:10.1016/j.neuroimage.2015.03.069 aging and Alzheimer’s disease. Alzheimers Dement. (1):117-128. doi:10.1002/ana.24844
20. Braak H, Alafuzoff I, Arzberger T, 2017;13(3):205-216. doi:10.1016/j.jalz.2016.08.005 48. Serrano-Pozo A, Qian J, Monsell SE, et al. Mild
Kretzschmar H, Del Tredici K. Staging of Alzheimer 35. Kang JH, Korecka M, Figurski MJ, et al; to moderate Alzheimer dementia with insufficient
disease–associated neurofibrillary pathology using Alzheimer’s Disease Neuroimaging Initiative. neuropathological changes. Ann Neurol. 2014;75
paraffin sections and immunocytochemistry. Acta The Alzheimer’s Disease Neuroimaging Initiative 2 (4):597-601. doi:10.1002/ana.24125
Neuropathol. 2006;112(4):389-404. doi:10.1007/ Biomarker Core: a review of progress and plans. 49. Tsai RM, Bejanin A, Lesman-Segev O, et al.
s00401-006-0127-z Alzheimers Dement. 2015;11(7):772-791. doi:10. 18
F-flortaucipir (AV-1451) tau PET in frontotemporal
21. Biel D, Brendel M, Rubinski A, et al; Alzheimer’s 1016/j.jalz.2015.05.003 dementia syndromes. Alzheimers Res Ther. 2019;11
Disease Neuroimaging Initiative (ADNI). Tau-PET 36. Donohue MC, Sperling RA, Salmon DP, et al; (1):13. doi:10.1186/s13195-019-0470-7
and in vivo Braak-staging as prognostic markers of Australian Imaging, Biomarkers, and Lifestyle 50. Makaretz SJ, Quimby M, Collins J, et al.
future cognitive decline in cognitively normal to Flagship Study of Ageing; Alzheimer’s Disease Flortaucipir tau PET imaging in semantic variant
demented individuals. Alzheimers Res Ther. 2021;13 Neuroimaging Initiative; Alzheimer’s Disease primary progressive aphasia. J Neurol Neurosurg
(1):137. doi:10.1186/s13195-021-00880-x Cooperative Study. The preclinical Alzheimer Psychiatry. 2018;89(10):1024-1031. doi:10.1136/jnnp-
22. Jagust WJ, Bandy D, Chen K, et al; Alzheimer’s cognitive composite: measuring amyloid-related 2017-316409
Disease Neuroimaging Initiative. The Alzheimer’s decline. JAMA Neurol. 2014;71(8):961-970. doi:10. 51. Josephs KA, Martin PR, Botha H, et al.
Disease Neuroimaging Initiative positron emission 1001/jamaneurol.2014.803 [18 F]AV-1451 tau-PET and primary progressive
tomography core. Alzheimers Dement. 2010;6(3): 37. Jack CR Jr, Wiste HJ, Weigand SD, et al. aphasia. Ann Neurol. 2018;83(3):599-611. doi:10.
221-229. doi:10.1016/j.jalz.2010.03.003 Age-specific and sex-specific prevalence of cerebral 1002/ana.25183
23. López-González FJ, Costoya-Sánchez A, β-amyloidosis, tauopathy, and neurodegeneration 52. Wolters EE, Papma JM, Verfaillie SCJ, et al.
Paredes-Pacheco J, Moscoso A, Silva-Rodríguez J, in cognitively unimpaired individuals aged 50-95 [18F]flortaucipir PET across various MAPT
Aguiar P; Alzheimer’s Disease Neuroimaging years: a cross-sectional study. Lancet Neurol. 2017; mutations in presymptomatic and symptomatic
Initiative. Impact of spill-in counts from off-target 16(6):435-444. doi:10.1016/S1474-4422(17)30077-7 carriers. Neurology. 2021;97(10):e1017-e1030.
regions on [18F]flortaucipir PET quantification. 38. Lowe VJ, Lundt ES, Albertson SM, et al. doi:10.1212/WNL.0000000000012448
Neuroimage. 2022;259:119396. doi:10.1016/j. Tau-positron emission tomography correlates with
neuroimage.2022.119396 53. Schaeverbeke J, Celen S, Cornelis J, et al.
neuropathology findings. Alzheimers Dement. Binding of [18F]AV1451 in post mortem brain slices
24. Thomas BA, Erlandsson K, Modat M, et al. 2020;16(3):561-571. doi:10.1016/j.jalz.2019.09.079 of semantic variant primary progressive aphasia
The importance of appropriate partial volume 39. Fleisher AS, Pontecorvo MJ, Devous MD Sr, patients. Eur J Nucl Med Mol Imaging. 2020;47(8):
correction for PET quantification in Alzheimer’s et al; A16 Study Investigators. Positron emission 1949-1960. doi:10.1007/s00259-019-04631-x
disease. Eur J Nucl Med Mol Imaging. 2011;38(6): tomography imaging with [18F]flortaucipir and
1104-1119. doi:10.1007/s00259-011-1745-9 54. Carlos AF, Tosakulwong N, Weigand SD, et al.
postmortem assessment of Alzheimer disease TDP-43 pathology effect on volume and flortaucipir
25. Thomas BA, Cuplov V, Bousse A, et al. PETPVC: neuropathologic changes. JAMA Neurol. 2020;77 uptake in Alzheimer’s disease. Alzheimers Dement.
a toolbox for performing partial volume correction (7):829-839. doi:10.1001/jamaneurol.2020.0528 2023;19(6):2343-2354. doi:10.1002/alz.12878
techniques in positron emission tomography. Phys 40. Moscoso A, Wren MC, Lashley T, et al. Imaging
Med Biol. 2016;61(22):7975-7993. doi:10.1088/ 55. Aksman LM, Oxtoby NP, Scelsi MA, et al.
tau pathology in Alzheimer’s disease with positron Tau-first subtype of Alzheimer’s disease
0031-9155/61/22/7975 emission tomography: lessons learned from consistently identified across in vivo and post
26. Baker SL, Maass A, Jagust WJ. Considerations imaging-neuropathology validation studies. Mol mortem studies. bioRxiv. Preprint posted online
and code for partial volume correcting [18F]-AV-1451 Neurodegener. 2022;17(1):39. doi:10.1186/s13024- December 19, 2020. doi:10.1101/2020.12.18.418004
tau PET data. Data Brief. 2017;15:648-657. doi:10. 022-00543-x
1016/j.dib.2017.10.024 56. Das SR, Xie L, Wisse LEM, et al; Alzheimer’s
41. Soleimani-Meigooni DN, Iaccarino L, La Joie R, Disease Neuroimaging Initiative. In vivo measures
27. Klunk WE, Koeppe RA, Price JC, et al. et al. 18F-flortaucipir PET to autopsy comparisons of tau burden are associated with atrophy in early
The Centiloid Project: standardizing quantitative in Alzheimer’s disease and other neurodegenerative Braak stage medial temporal lobe regions in
amyloid plaque estimation by PET. Alzheimers diseases. Brain. 2020;143(11):3477-3494. doi:10. amyloid-negative individuals. Alzheimers Dement.
Dement. 2015;11(1):1-15.e1, 4. doi:10.1016/j.jalz.2014. 1093/brain/awaa276 2019;15(10):1286-1295. doi:10.1016/j.jalz.2019.
07.003 42. Lowe VJ, Curran G, Fang P, et al. 05.009
28. Dale AM, Fischl B, Sereno MI. Cortical An autoradiographic evaluation of AV-1451 Tau PET 57. Weigand AJ, Maass A, Eglit GL, Bondi MW.
surface-based analysis, I: segmentation and surface in dementia. Acta Neuropathol Commun. 2016;4(1): What’s the cut-point? a systematic investigation of
reconstruction. Neuroimage. 1999;9(2):179-194. 58. doi:10.1186/s40478-016-0315-6 tau PET thresholding methods. Alzheimers Res Ther.
doi:10.1006/nimg.1998.0395 43. Marquié M, Siao Tick Chong M, 2022;14(1):49. doi:10.1186/s13195-022-00986-w
29. Fischl B, Sereno MI, Dale AM. Cortical Antón-Fernández A, et al. [F-18]-AV-1451 binding
surface-based analysis, II: inflation, flattening, and a
jamaneurology.com (Reprinted) JAMA Neurology Published online August 14, 2023 E11
Downloaded From: https://jamanetwork.com/ on 08/29/2023
You might also like
- The Lightworker's HandbookDocument58 pagesThe Lightworker's HandbookEvgeniq Marcenkova100% (9)
- RZR XP Turbo With EVAP Wiring DiagramDocument3 pagesRZR XP Turbo With EVAP Wiring Diagram张连杉No ratings yet
- The Henna Page "How-To" Mix HennaDocument44 pagesThe Henna Page "How-To" Mix HennaCatherine Cartwright-Jones93% (14)
- Jamaneurology Costoyasnchez 2023 Oi 230055 1695756376.47025Document11 pagesJamaneurology Costoyasnchez 2023 Oi 230055 1695756376.47025Elton MatsushimaNo ratings yet
- Tau PET and Multimodal Brain Imaging in Patients at Risk F - 2019 - NeuroImageDocument13 pagesTau PET and Multimodal Brain Imaging in Patients at Risk F - 2019 - NeuroImageBruno MañonNo ratings yet
- Sankar Et Al-2017-Human Brain MappingDocument22 pagesSankar Et Al-2017-Human Brain Mappingdess101No ratings yet
- A Review of Brain and Pituitary Gland MRI Findings in Patients With Ataxia and HypogonadismDocument18 pagesA Review of Brain and Pituitary Gland MRI Findings in Patients With Ataxia and HypogonadismyusranurparlakNo ratings yet
- Neurobiology of Disease: ReviewDocument13 pagesNeurobiology of Disease: ReviewValentina BusinaroNo ratings yet
- Changes in Plasma Amyloid and Tau in ADocument16 pagesChanges in Plasma Amyloid and Tau in AeastareaNo ratings yet
- 3917 FullDocument15 pages3917 FullWesleyNo ratings yet
- Neurobiology of AgingDocument11 pagesNeurobiology of AgingLuis GómezNo ratings yet
- Molekuler DiagnostikDocument19 pagesMolekuler DiagnostikAulia HudaNo ratings yet
- Association of Plasma Total Tau Level With Cognitive Decline and Risk of Mild Cognitive Impairment or Dementia in The Mayo Clinic Study On AgingDocument8 pagesAssociation of Plasma Total Tau Level With Cognitive Decline and Risk of Mild Cognitive Impairment or Dementia in The Mayo Clinic Study On AgingeastareaNo ratings yet
- SPECTand PETin Alzheimers ValotasiouDocument14 pagesSPECTand PETin Alzheimers ValotasiouTony AndersonNo ratings yet
- Current Concepts On Epilepsy Management in Tuberous SclerosisDocument10 pagesCurrent Concepts On Epilepsy Management in Tuberous Sclerosiselmon patadunganNo ratings yet
- Reduced Corticostriatal Functional Connectivity in Temporomandibular DisordersDocument10 pagesReduced Corticostriatal Functional Connectivity in Temporomandibular DisordersCata MirandaNo ratings yet
- Corticalstriatal Interactions in The Generation of Tic-Like Behaviors After Local Striatal DesinhibitionDocument7 pagesCorticalstriatal Interactions in The Generation of Tic-Like Behaviors After Local Striatal DesinhibitionCornelia ParaipanNo ratings yet
- Jamaneurology Leuzy 2023 Oi 230023 1686342376.28349Document10 pagesJamaneurology Leuzy 2023 Oi 230023 1686342376.28349MA MANo ratings yet
- tmpCB2D TMPDocument10 pagestmpCB2D TMPFrontiersNo ratings yet
- Alzheimer and AcetylcholineDocument14 pagesAlzheimer and Acetylcholinefy fyNo ratings yet
- 2023 - Updated Clinical Recommendations For The Management of Tuberous Sclerosis Complex Associated EpilepsyDocument10 pages2023 - Updated Clinical Recommendations For The Management of Tuberous Sclerosis Complex Associated EpilepsyilonaskorinNo ratings yet
- 2014 Galeano P Frontiers in Behavioural Neuroscience 8 321Document15 pages2014 Galeano P Frontiers in Behavioural Neuroscience 8 321Eduardo Blanco CalvoNo ratings yet
- WNL 2022 201671Document14 pagesWNL 2022 201671PeyepeyeNo ratings yet
- Tinnitus fMRI Study 2011Document9 pagesTinnitus fMRI Study 2011Mike F MartelliNo ratings yet
- Complex I Klinisch ReviewDocument11 pagesComplex I Klinisch Reviewsaskiakoene84No ratings yet
- 10.1016/j.jns.2023.121122: Abstracts / Journal of The Neurological Sciences 455 (2023) 121048 38Document1 page10.1016/j.jns.2023.121122: Abstracts / Journal of The Neurological Sciences 455 (2023) 121048 38Amin AminiNo ratings yet
- Aittokallio, Virkki, Polo - 2009 - Understanding Sleep-Disordered Breathing Through Mathematical ModellingDocument11 pagesAittokallio, Virkki, Polo - 2009 - Understanding Sleep-Disordered Breathing Through Mathematical ModellingFGR2711No ratings yet
- 3 ArticleDocument59 pages3 ArticleAlbert PierssonNo ratings yet
- Keller Et Al 2011 EpilepsiaDocument10 pagesKeller Et Al 2011 EpilepsiaLaura Gonzalez TamayoNo ratings yet
- Clin Nuc Med 2015. EncefalitisDocument3 pagesClin Nuc Med 2015. EncefalitisPuig CozarNo ratings yet
- 2020 - Neuroradiology - Basal GGL and Thalamus-2Document41 pages2020 - Neuroradiology - Basal GGL and Thalamus-2Andreea BanicaNo ratings yet
- Zns 2449Document14 pagesZns 2449Bogdan A. GireadăNo ratings yet
- Association Neurofilament and Alzheimer Progression Mattsson2019Document9 pagesAssociation Neurofilament and Alzheimer Progression Mattsson2019eastareaNo ratings yet
- 9877 ArticleText 54433 1 10 20210831Document7 pages9877 ArticleText 54433 1 10 20210831xnpghb5ppfNo ratings yet
- Differential Diagnosis of Cerebellar Atrophy in Childhood An UpdateDocument13 pagesDifferential Diagnosis of Cerebellar Atrophy in Childhood An UpdatehengkileonardNo ratings yet
- Long-Term Prognosis After Childhood Convulsive Status Epilepticus: A Prospective Cohort StudyDocument9 pagesLong-Term Prognosis After Childhood Convulsive Status Epilepticus: A Prospective Cohort Studynadia pramitha157No ratings yet
- Temporal Lobe Epilepsy With Amygdala Enlargement: A Subtype of Temporal Lobe EpilepsyDocument8 pagesTemporal Lobe Epilepsy With Amygdala Enlargement: A Subtype of Temporal Lobe EpilepsyRavi ChandraNo ratings yet
- Citicoline in Intracerebral Haemorrhage: A Double-Blind, Randomized, Placebo-Controlled, Multi-Centre Pilot StudyDocument6 pagesCiticoline in Intracerebral Haemorrhage: A Double-Blind, Randomized, Placebo-Controlled, Multi-Centre Pilot StudyTiti SulistiowatiNo ratings yet
- J Clinph 2013 03 031Document7 pagesJ Clinph 2013 03 031Andres Rojas JerezNo ratings yet
- Martin 2014 AutismDocument8 pagesMartin 2014 AutismJortegloriaNo ratings yet
- Modeling Alzheimer's Disease in Transgenic RatsDocument11 pagesModeling Alzheimer's Disease in Transgenic RatsHümay ÜnalNo ratings yet
- Computers in Biology and Medicine: SciencedirectDocument9 pagesComputers in Biology and Medicine: SciencedirectAnthony ParkNo ratings yet
- Alzheimer Mechanisms and Therapeutic StrategiesDocument19 pagesAlzheimer Mechanisms and Therapeutic StrategiesArtemis LiNo ratings yet
- Brain Glucose Metabolism and Its RelatioDocument4 pagesBrain Glucose Metabolism and Its RelatioSusana Nuñez RipollNo ratings yet
- ClinicalCharacteristicsAndProgrognostic AstrocytomaDocument12 pagesClinicalCharacteristicsAndProgrognostic AstrocytomaDesty PurnamasariNo ratings yet
- Jamaneurology Smith 2023 Oi 230028 1688145189.33804Document8 pagesJamaneurology Smith 2023 Oi 230028 1688145189.33804Yenny MaharaniNo ratings yet
- Acidos GrasosDocument14 pagesAcidos GrasoscristhianldsNo ratings yet
- Alzheimer's DiseaseDocument14 pagesAlzheimer's DiseaseM BORQUEZ QUINTASNo ratings yet
- EA Lancet 2021Document14 pagesEA Lancet 2021Felipe SennNo ratings yet
- Ramesh 2018Document11 pagesRamesh 2018Ricardo MedinaNo ratings yet
- J 1528-1167 2008 01506EDWDocument8 pagesJ 1528-1167 2008 01506EDWheribertoNo ratings yet
- Early Diagnosis and Prevention of Repetitive Strain Injury Induced Carpaltunnel Syndrome Among Computer Users 2471 2701 1000188Document2 pagesEarly Diagnosis and Prevention of Repetitive Strain Injury Induced Carpaltunnel Syndrome Among Computer Users 2471 2701 1000188febby astariNo ratings yet
- 2012 Theneuroprotectiveroleof TERTviaanantiapoptoticDocument6 pages2012 Theneuroprotectiveroleof TERTviaanantiapoptoticLoic MCNo ratings yet
- High-Dose Erythropoietin For Asphyxia andDocument8 pagesHigh-Dose Erythropoietin For Asphyxia andFer45No ratings yet
- Is Electrocochleography Still Helpful in Early Diagnosis of Meniere Disease?Document5 pagesIs Electrocochleography Still Helpful in Early Diagnosis of Meniere Disease?nandaanggiNo ratings yet
- Altered Brain Energy Metabolism Related To Astrocytes in Alzheimer's DiseaseDocument33 pagesAltered Brain Energy Metabolism Related To Astrocytes in Alzheimer's DiseaseКолевска МиленаNo ratings yet
- Cortical Thickness Gray White Matter Contrast and Intracortica - 2023 - PsychiDocument9 pagesCortical Thickness Gray White Matter Contrast and Intracortica - 2023 - Psychitito syahjihadNo ratings yet
- Screening Tools PDFDocument8 pagesScreening Tools PDFNoemia ChumboNo ratings yet
- Evaluation of Electroretinography ERG Parameters As A BiomarkerDocument8 pagesEvaluation of Electroretinography ERG Parameters As A Biomarkerabsabravo4No ratings yet
- MRI in Evaluating AdenocarsinomaDocument5 pagesMRI in Evaluating AdenocarsinomaAnggit Adhi PrasetyaNo ratings yet
- Severity Assessment and Scoring For Neurosurgical Models in RodentsDocument11 pagesSeverity Assessment and Scoring For Neurosurgical Models in RodentsKazhi SujudNo ratings yet
- PEATE Doença de MeniereDocument8 pagesPEATE Doença de MeniereLuanna CarlosNo ratings yet
- Adams and Victor Disease of Muscle-2-25Document24 pagesAdams and Victor Disease of Muscle-2-25Yenny MaharaniNo ratings yet
- Epilepsia - 2018 - GibbsDocument5 pagesEpilepsia - 2018 - GibbsYenny MaharaniNo ratings yet
- Jamaneurology Erickson 2023 Oi 230049 1689611082.26721Document11 pagesJamaneurology Erickson 2023 Oi 230049 1689611082.26721Yenny MaharaniNo ratings yet
- The Spinal Cord TERJEMAHANDocument14 pagesThe Spinal Cord TERJEMAHANYenny MaharaniNo ratings yet
- Epilepsia - 2023 - SmithDocument9 pagesEpilepsia - 2023 - SmithYenny MaharaniNo ratings yet
- Jamaneurology Ayroza Galvo Ribeiro Gomes 2023 BR 230003 1691038081.02612Document7 pagesJamaneurology Ayroza Galvo Ribeiro Gomes 2023 BR 230003 1691038081.02612Yenny MaharaniNo ratings yet
- FigureDocument1 pageFigureYenny MaharaniNo ratings yet
- Kim 2023 Oi 230173 1678906601.17837Document12 pagesKim 2023 Oi 230173 1678906601.17837Yenny MaharaniNo ratings yet
- Strokeaha 122 042053Document9 pagesStrokeaha 122 042053Yenny MaharaniNo ratings yet
- Epilepsia - 2023 - LarssonDocument8 pagesEpilepsia - 2023 - LarssonYenny MaharaniNo ratings yet
- Have Climbed Highest MountainsDocument1 pageHave Climbed Highest MountainsYenny MaharaniNo ratings yet
- GearsDocument246 pagesGearsVinayak AryanNo ratings yet
- Aakash Resume FinalDocument2 pagesAakash Resume FinalAAKASH DHIMANNo ratings yet
- Chapters 1, 2 & 6Document95 pagesChapters 1, 2 & 6Mwizukanji NakambaNo ratings yet
- Textbook Prism and Lens Making A Textbook For Optical Glassworkers Second Edition Frank Twyman Ebook All Chapter PDFDocument53 pagesTextbook Prism and Lens Making A Textbook For Optical Glassworkers Second Edition Frank Twyman Ebook All Chapter PDFleon.beattie194100% (6)
- COVID 19 PP Original 1Document20 pagesCOVID 19 PP Original 1Anthony UjeneNo ratings yet
- Essentials Lab Resource Guide 12 22Document67 pagesEssentials Lab Resource Guide 12 22Aijeleth Shahar Gunay AwacayNo ratings yet
- Ademco Vista48laDocument80 pagesAdemco Vista48lajoelNo ratings yet
- Stinger 3470Document8 pagesStinger 3470allen_renziNo ratings yet
- Oxygen Concentrator 7F-5 User's ManualDocument9 pagesOxygen Concentrator 7F-5 User's ManualJunx TripoliNo ratings yet
- Area of A CircleDocument2 pagesArea of A CircleHandri eko100% (1)
- Red PandaDocument24 pagesRed PandaDaphne Tan 丽文50% (2)
- Atex Directive in A NutshellDocument4 pagesAtex Directive in A NutshellAnonymous 96SYLheENo ratings yet
- Phontech Ics 6200Document193 pagesPhontech Ics 6200danh voNo ratings yet
- LanguageDocument8 pagesLanguageAlysson CamposNo ratings yet
- Call For Papers-International Journal of Wireless & Mobile Networks (IJWMN)Document2 pagesCall For Papers-International Journal of Wireless & Mobile Networks (IJWMN)John BergNo ratings yet
- Well Stimulation ServicesDocument6 pagesWell Stimulation ServicesAfzal AktharNo ratings yet
- Diagnostic Imaging Pathways ArticleDocument10 pagesDiagnostic Imaging Pathways ArticleSuhayatra PutraNo ratings yet
- Anime Neon Vibes Marketing PlanDocument62 pagesAnime Neon Vibes Marketing PlanAL - HusainNo ratings yet
- Intech Brachytherapy Shielding Materials Guide 2021-01Document3 pagesIntech Brachytherapy Shielding Materials Guide 2021-01Kevin DrummNo ratings yet
- 13.8KV Unit Substation-InstallationDocument1 page13.8KV Unit Substation-InstallationMuhammad IrfanNo ratings yet
- Questions ExplanationDocument63 pagesQuestions ExplanationnomintmNo ratings yet
- Jurnal EntropionDocument7 pagesJurnal EntropionOgiesilaenNo ratings yet
- The Long Term Family Survival Course v5Document323 pagesThe Long Term Family Survival Course v5António Manuel de MoraisNo ratings yet
- Asset Life Cycle ManagementDocument14 pagesAsset Life Cycle ManagementSheeba Mathew100% (1)
- Electrochemistry FR Worksheet Answers Key PDFDocument22 pagesElectrochemistry FR Worksheet Answers Key PDFEmily toaNo ratings yet
- BMW 7 Series (G12) 2016+ Technical Doc - Owner's ManualDocument314 pagesBMW 7 Series (G12) 2016+ Technical Doc - Owner's ManualPhan Văn100% (1)
- Group 4: Diet For Healthy Teath BonesDocument26 pagesGroup 4: Diet For Healthy Teath Bonesknowledge chanall chanallNo ratings yet