Professional Documents
Culture Documents
Covid Test Results
Uploaded by
bhatia9290 ratings0% found this document useful (0 votes)
10 views1 pageParul Raza, a 58-year-old female, was tested for SARS-CoV-2 and the results were negative, meaning the virus was not detected. The test was developed by LabCorp Laboratories and has been authorized by the FDA under an Emergency Use Authorization. When diagnostic testing is negative, the possibility of a false negative result should be considered based on recent exposures and symptoms. The sample was collected on May 9, 2021 and reported on the same day.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentParul Raza, a 58-year-old female, was tested for SARS-CoV-2 and the results were negative, meaning the virus was not detected. The test was developed by LabCorp Laboratories and has been authorized by the FDA under an Emergency Use Authorization. When diagnostic testing is negative, the possibility of a false negative result should be considered based on recent exposures and symptoms. The sample was collected on May 9, 2021 and reported on the same day.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
10 views1 pageCovid Test Results
Uploaded by
bhatia929Parul Raza, a 58-year-old female, was tested for SARS-CoV-2 and the results were negative, meaning the virus was not detected. The test was developed by LabCorp Laboratories and has been authorized by the FDA under an Emergency Use Authorization. When diagnostic testing is negative, the possibility of a false negative result should be considered based on recent exposures and symptoms. The sample was collected on May 9, 2021 and reported on the same day.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
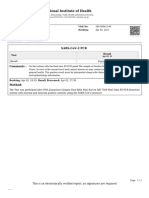
LabCorp PATIENT INFORMATION REPORT STATUS: FINAL
SPECIMEN INFORMATION Raza, Parul
ORDERING PHYSICIAN
ACCOUNT NO: 31181380 DOB: May 14, 1962
Abraham, M.
SPECIMEN: 12896840880 AGE: 58
NPI: 1740299965
REQUISITION: 74152320 GENDER: Female
CLIENT INFORMATION
Lab ref no: FASTING: Unknown
Walgreens COVID-19
PATIENT ID: 74152320
COLLECTED: 05/09/2021 08:30AM PDT
RECEIVED: 05/08/2021 Clinical Info:
REPORTED: 05/09/2021 01:06PM ET
Test Name Result Flag Reference Range Lab
SARS-CoV-2, NAA
SARS-CoV-2, NAA Not Detected NORMAL Not Detected 01
This nucleic acid amplification test was developed and its performance
characteristics determined by LabCorp Laboratories. Nucleic acid
amplification tests include RT-PCR and TMA. This test has not been
FDA cleared or approved. This test has been authorized by FDA under
an Emergency Use Authorization (EUA). This test is only authorized
for the duration of time the declaration that circumstances exist
justifying the authorization of the emergency use of in vitro
diagnostic tests for detection of SARS-CoV-2 virus and/or diagnosis
of COVID-19 infection under section 564(b)(1) of the Act, 21 U.S.C.
360bbb-3(b) (1), unless the authorization is terminated or revoked
sooner.
When diagnostic testing is negative, the possibility of a false
negative result should be considered in the context of a patient's
recent exposures and the presence of clinical signs and symptoms
consistent with COVID-19. An individual without symptoms of COVID-19
and who is not shedding SARS-CoV-2 virus would expect to have a
negative (not detected) result in this assay.
SARS-CoV-2, NAA 2 DAY TAT
SARS-CoV-2, NAA 2 DAY TAT Performed NORMAL 02
Performing Laboratory Information:
01: Esoterix Genetic Laboratories, 3400 Computer Drive, Westborough MA, 015811771, phone: 800-
255-7357, Director: PhD Bernice A Allitto
02: LabCorp Raritan, 69 First Avenue, Raritan NJ, 088691800, phone: 800-631-5250, Director: MD
Araceli B Reyes
1 of 1
You might also like
- Brown, Joielle 08/24/1983 Patient Report: Ordered Items: Sars-Cov-2, NaaDocument1 pageBrown, Joielle 08/24/1983 Patient Report: Ordered Items: Sars-Cov-2, NaajoiNo ratings yet
- Alejadro PCRDocument1 pageAlejadro PCRFirst Level Consulting SACNo ratings yet
- Test Name Result Flag Reference Range Lab: Patient InformationDocument1 pageTest Name Result Flag Reference Range Lab: Patient InformationRobNo ratings yet
- Test Name Result Flag Reference Range Lab: Abraham, Andrew NPI: 1184883993Document2 pagesTest Name Result Flag Reference Range Lab: Abraham, Andrew NPI: 1184883993SandraNo ratings yet
- Test Name Result Flag Reference Range Lab: Patient InformationDocument1 pageTest Name Result Flag Reference Range Lab: Patient InformationosmolympiaNo ratings yet
- Candidiasis - Causes, Symptoms, Treatment, DiagnosisDocument5 pagesCandidiasis - Causes, Symptoms, Treatment, DiagnosisChaiwa JustineNo ratings yet
- SOP For Procurement of CultureDocument5 pagesSOP For Procurement of Culturegreen solution100% (2)
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusAtharNo ratings yet
- Chapter 4Document27 pagesChapter 4Blessy Martin100% (1)
- Department of Molecular Biology. The Automotive Reasearch India - Covid 19 RT PCR - Pune - Fy2122 Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. The Automotive Reasearch India - Covid 19 RT PCR - Pune - Fy2122 Test Name Result Unit Bio. Ref. Range MethodanilsgaikwadNo ratings yet
- Introduction To MycologyDocument66 pagesIntroduction To MycologyHerlinaNababanNo ratings yet
- Impetigo PathophysiologyDocument1 pageImpetigo PathophysiologyCesar Arada100% (1)
- Bio-Rad Rapid'Salmonella Test ProsedürüDocument5 pagesBio-Rad Rapid'Salmonella Test ProsedürügokhanNo ratings yet
- Test Name Result Flag Reference Range Lab: Patient InformationDocument1 pageTest Name Result Flag Reference Range Lab: Patient InformationRaquel LujanNo ratings yet
- Test Name Result Flag Reference Range Lab: Patient InformationDocument1 pageTest Name Result Flag Reference Range Lab: Patient InformationroxanaNo ratings yet
- Test Name Result Flag Reference Range Lab: Patient InformationDocument1 pageTest Name Result Flag Reference Range Lab: Patient InformationAngel ManuelNo ratings yet
- RapidCare - RT PCR - September 5th 3Document1 pageRapidCare - RT PCR - September 5th 3দীপা পালNo ratings yet
- Srinanda SarkarDocument1 pageSrinanda SarkarBadsha MondalNo ratings yet
- Department of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodPrantik MaityNo ratings yet
- Sars-Cov-2 RT PCR Testing: Test Description Method ResultDocument1 pageSars-Cov-2 RT PCR Testing: Test Description Method ResultMAYUR PATELNo ratings yet
- AntigenDocument2 pagesAntigenMisty Michelle PedrosaNo ratings yet
- Uph22-16161 - Tamoria, Ariel Marcelino BautistaDocument1 pageUph22-16161 - Tamoria, Ariel Marcelino BautistaAriel Marcelino Bautista TamoriaNo ratings yet
- AntigenDocument2 pagesAntigenMisty Michelle PedrosaNo ratings yet
- Sars-Cov-2 (Causative Agent of Covid-19 Viral Rna Not DetectedDocument1 pageSars-Cov-2 (Causative Agent of Covid-19 Viral Rna Not DetectedKaoruTecsonNo ratings yet
- Department of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodManoj NainNo ratings yet
- LG23 566175Document1 pageLG23 566175Airo Nikko SolpicoNo ratings yet
- Department of Molecular Biology and Cytogenetics:: Mr. Banavathu Gopi Kiran NaikDocument1 pageDepartment of Molecular Biology and Cytogenetics:: Mr. Banavathu Gopi Kiran NaikGopi Kiran NaikNo ratings yet
- Rd-cvd19-Mohd Fakhrur Razi Bin Mohamad Zin-6aoxfic7Document1 pageRd-cvd19-Mohd Fakhrur Razi Bin Mohamad Zin-6aoxfic7Huzaifah ZinNo ratings yet
- Sars-Cov-2 Real Time PCR (Qualitative: Molecular LabDocument1 pageSars-Cov-2 Real Time PCR (Qualitative: Molecular LabMohammad KhalidNo ratings yet
- Aed2020-27745 MR - Tejashwin Ravishankar 129334Document1 pageAed2020-27745 MR - Tejashwin Ravishankar 129334sadhanaNo ratings yet
- LabResultTempPDF CJ0304865Document2 pagesLabResultTempPDF CJ0304865Jahred EstebanNo ratings yet
- PCR My - Wan Nur Afiyah - 8mayDocument1 pagePCR My - Wan Nur Afiyah - 8mayEvie SuriNo ratings yet
- Rahul SharmaDocument3 pagesRahul Sharmaarunitsaraogi7No ratings yet
- Covid-19 by Real Time RT PCRDocument1 pageCovid-19 by Real Time RT PCRArun AntonyNo ratings yet
- Marcelino, Christian - PCRDocument1 pageMarcelino, Christian - PCREhmMarcelinoNo ratings yet
- MR Salman Ali: Molecular LabDocument1 pageMR Salman Ali: Molecular LabMohammad KhalidNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) Virusshoaib chNo ratings yet
- Passport First Name Last Name DOB GenderDocument1 pagePassport First Name Last Name DOB GenderCODE 88No ratings yet
- Test Name Result Flag Reference Range Lab: Patient InformationDocument1 pageTest Name Result Flag Reference Range Lab: Patient InformationSefatullahNo ratings yet
- Philippine Red Cross Molecular Laboratory: Covid-19 RT-PCR Test Report & CertificationDocument1 pagePhilippine Red Cross Molecular Laboratory: Covid-19 RT-PCR Test Report & CertificationChris-Goldie LorezoNo ratings yet
- MyChart - Test DetailsDocument2 pagesMyChart - Test DetailsMaría RepettoNo ratings yet
- T2100132822 P2100112050 0 T2100132822 Telecare 0 19811126 $ml-DefaultDocument1 pageT2100132822 P2100112050 0 T2100132822 Telecare 0 19811126 $ml-DefaultRoyzen VillaruelNo ratings yet
- Mariano Marcos Memorial Hospital and Medical Center: Molecular Biology LaboratoryDocument1 pageMariano Marcos Memorial Hospital and Medical Center: Molecular Biology LaboratoryJasper Trinidad BonnaoNo ratings yet
- Molecular Biology: Verdad, Marvin AlmaidaDocument1 pageMolecular Biology: Verdad, Marvin AlmaidaMarvin VerdadNo ratings yet
- Covid-19 by Real Time RT PCRDocument1 pageCovid-19 by Real Time RT PCRArun AntonyNo ratings yet
- Sars-Cov2 (Covid-19) Real Time RT PCR Test: Positive and Negative Controls For All The Three Genes Were SatisfactoryDocument1 pageSars-Cov2 (Covid-19) Real Time RT PCR Test: Positive and Negative Controls For All The Three Genes Were Satisfactorygowtham thakutNo ratings yet
- Report ViewerDocument1 pageReport Viewervoldemort killerNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range Methodmaneesh babuNo ratings yet
- Ma'ruf SabilanDocument1 pageMa'ruf SabilanManga MinNo ratings yet
- 21081916232464@gao, Shan - 8021148425Document1 page21081916232464@gao, Shan - 8021148425MARIA CRISTINA DE PAZNo ratings yet
- Patients Profile: Not Detected NegativeDocument2 pagesPatients Profile: Not Detected NegativeELLIE JAMES PLACIONo ratings yet
- Valenzuela James BacudDocument1 pageValenzuela James BacudJames B ValenzuelaNo ratings yet
- T2200053158 P2200045869 0 T2200053158 62 0 20000115 $ml-DefaultDocument1 pageT2200053158 P2200045869 0 T2200053158 62 0 20000115 $ml-DefaultShaira BungayNo ratings yet
- Sars-Cov-2/Covid-19 Test Report: Amendments / CorrectionsDocument1 pageSars-Cov-2/Covid-19 Test Report: Amendments / CorrectionsSebastian PradaNo ratings yet
- Aragaw 206714-1 364272Document1 pageAragaw 206714-1 364272zeine omerNo ratings yet
- Test Description Results Units Reference Range Abnormal Lab: Moutou, MathieuDocument2 pagesTest Description Results Units Reference Range Abnormal Lab: Moutou, MathieuMathieu François MoutouNo ratings yet
- MOTION AC en - US 864001146526 1656423310836Document1 pageMOTION AC en - US 864001146526 1656423310836EmanuelleNo ratings yet
- PKD 20210830426Document1 pagePKD 20210830426sabithsabzinNo ratings yet
- Sars-Cov-2 (Causative Agent of Covid-19 Viral Rna Not DetectedDocument1 pageSars-Cov-2 (Causative Agent of Covid-19 Viral Rna Not DetectedKaoruTecsonNo ratings yet
- National Institute of Health: Sars-Cov-2 PCRDocument1 pageNational Institute of Health: Sars-Cov-2 PCRDRSM QAUNo ratings yet
- Sars-Cov-2 RT PCR Testing: Test Description Method ResultDocument1 pageSars-Cov-2 RT PCR Testing: Test Description Method ResultRitesh RanjanNo ratings yet
- Laboratory Result Form: Pontilar, Gretchel CondinoDocument1 pageLaboratory Result Form: Pontilar, Gretchel CondinoGretchel PontilarNo ratings yet
- Department of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodDocument4 pagesDepartment of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range Methodsourabhshrivastava80No ratings yet
- L24198772 (TS115886) : 2021:VI81463R:: Saqib, ShahidDocument2 pagesL24198772 (TS115886) : 2021:VI81463R:: Saqib, Shahidsidra anjumNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodGirija Prasad SwainNo ratings yet
- Evidence-Based Research in Ayurveda Against COVID-19 in Compliance with Standardized Protocols and PracticesFrom EverandEvidence-Based Research in Ayurveda Against COVID-19 in Compliance with Standardized Protocols and PracticesNo ratings yet
- Emergence of MDR-TBDocument18 pagesEmergence of MDR-TBAmor SantiagoNo ratings yet
- CGH202012022479 Lab-2020-0411918 Laboratory Covid-Pcr-TestDocument2 pagesCGH202012022479 Lab-2020-0411918 Laboratory Covid-Pcr-TestJosa Camille BungayNo ratings yet
- Parker 2001Document6 pagesParker 2001Chinasa EkejiubaNo ratings yet
- Entamoeba Coli NotesDocument4 pagesEntamoeba Coli NotesAnge OuedraogoNo ratings yet
- Poultry DiseasesDocument13 pagesPoultry DiseasesSajjad LaghariNo ratings yet
- Composición Extracto de LevaduraDocument1 pageComposición Extracto de LevaduraJuank González TéllezNo ratings yet
- A. True (Your Answer)Document7 pagesA. True (Your Answer)Tony DawaNo ratings yet
- Sulfonamides: Miss Preeti Verma Assistant Professor Faculty of Pharmaceutical Sciences, Rama University, Kanpur, U.PDocument17 pagesSulfonamides: Miss Preeti Verma Assistant Professor Faculty of Pharmaceutical Sciences, Rama University, Kanpur, U.PYash SinghNo ratings yet
- LB 1 Master 2018 OkDocument13,660 pagesLB 1 Master 2018 OkGha AnkNo ratings yet
- Reuben Et Al - 2019. Multispecies Interactions in Biofilms and ImplicationsDocument15 pagesReuben Et Al - 2019. Multispecies Interactions in Biofilms and ImplicationsAna Paula BertãoNo ratings yet
- 11 Oxirane GC MSDocument6 pages11 Oxirane GC MSCHRISTIAN FELIPE JIMENEZ MURILLONo ratings yet
- Vector DiseaseDocument2 pagesVector DiseasenallurihpNo ratings yet
- The Solution: TICKPLEX®: Multiplex Format: Multi-Functional: Highly Sensitive DetectionDocument2 pagesThe Solution: TICKPLEX®: Multiplex Format: Multi-Functional: Highly Sensitive DetectionTsvetlena IlievaNo ratings yet
- Dodi Daftar PustakaDocument3 pagesDodi Daftar PustakaToni PinemNo ratings yet
- Herpes Fact Sheet Lowres 2010Document2 pagesHerpes Fact Sheet Lowres 2010Thomas ThrashNo ratings yet
- 34Document2 pages34Muhammad NaveedNo ratings yet
- AIDS Case StudyDocument4 pagesAIDS Case StudyAlexandria BrooksNo ratings yet
- Lecture 7 Nematodes Part 2 New 2023Document19 pagesLecture 7 Nematodes Part 2 New 2023ayaessam392002No ratings yet
- Peace Corps Vaccine Administration Schedule - TG 300 Medical Technical Guideline 300 - July 2008Document4 pagesPeace Corps Vaccine Administration Schedule - TG 300 Medical Technical Guideline 300 - July 2008Accessible Journal Media: Peace Corps Documents100% (1)
- Microbiology and Parasitology Recall QuestionsDocument3 pagesMicrobiology and Parasitology Recall QuestionsKenneth MiguelNo ratings yet
- Price List HNA - All Share - Updated - 100123Document1 pagePrice List HNA - All Share - Updated - 100123nurul kusumaNo ratings yet
- CH 13 Burtons Diagnosing Infectious DiseasesDocument17 pagesCH 13 Burtons Diagnosing Infectious DiseasesedemcantosumjiNo ratings yet
- Genus CorynebacteriumDocument33 pagesGenus CorynebacteriumChin MartinzNo ratings yet
- Staphylococcal Food PoisoningDocument18 pagesStaphylococcal Food PoisoningMuhammad Hafidz Bin HasanNo ratings yet