Professional Documents
Culture Documents
Kim 2008
Uploaded by
JEAN CERNA SOLISOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Kim 2008
Uploaded by
JEAN CERNA SOLISCopyright:
Available Formats
REVIEWS
Mechanisms of microbial traversal of
the blood–brain barrier
Kwang Sik Kim
Abstract | Central nervous system (CNS) infections continue to be an important cause of
morbidity and mortality. Microbial invasion and traversal of the blood–brain barrier is a
prerequisite for CNS infections. Pathogens can cross the blood–brain barrier transcellularly,
paracellularly and/or in infected phagocytes (the so-called Trojan-horse mechanism).
Consequently, pathogens can cause blood–brain barrier dysfunction, including increased
permeability, pleocytosis and encephalopathy. A more complete understanding of the
microbial–host interactions that are involved in microbial traversal of the blood–brain
barrier and the associated barrier dysfunction should help to develop new strategies to
prevent CNS infections.
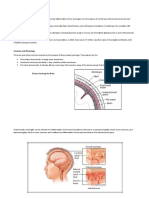
Astrocyte
Central nervous system (CNS) infections continue protects the brain from any microorganisms and toxins
A star-shaped glial cell that to be an important cause of morbidity and mortality that are circulating in the blood. Brain microvascular
supports the tissue of the throughout the world. For example, bacterial menin- endothelial cells have distinctive features, such as tight
central nervous system. gitis is one of the top ten causes of infection-related junctions and low rates of pinocytosis8–11. Astrocytes
deaths worldwide 1. Morbidity and mortality rates and pericytes help maintain the barrier property of
Pericyte
vary, however, depending on the age and location of brain microvascular endothelial cells, but their con-
A cell that is found around
capillaries and is related to
the patient and the causative organism. Patient groups tributions to microbial traversal of the barrier remain
smooth muscle cells. Pericytes who are at risk of high rates of morbidity and mor- incompletely understood. For example, the contribu-
surround the endothelium as tality include newborns, the elderly and those living tions of astrocytes and pericytes to Escherichia coli
single cells. Association with in developing countries, and the infections that have translocation of the barrier are minimal. By contrast,
pericytes reduces endothelial
apoptosis and stabilizes the
higher rates of morbidity and mortality are those astrocytes, together with microglial cells, regulate the
vasculature. caused by Gram-negative bacilli and Streptococcus recruitment of infiltrating haematogenous cells12 and
pneumoniae2–5. A major contributing factor to the lack might affect the translocation of some microorganisms.
Pinocytosis of preventive measures against CNS infections is our In addition, the antimicrobial activities of astrocytes
The cellular uptake of incomplete understanding of their pathogenesis 6,7. (such as indoleamine 2,3‑dioxygenase activity) and the
extracellular fluid. Involves the
Almost all microorganisms that are pathogenic to modulation of signal-transduction pathways in brain
formation of caveolae by the
cell membrane that pinch off to
humans have the potential to penetrate the CNS, but endothelial cells by pericytes might affect microbial
form vesicles in the cytoplasm. it is still unclear why a comparatively small number traversal of the barrier13,14.
of microbial pathogens account for most cases of CNS Studies of microbial traversal of the blood–brain
Microglial cell infection in humans. barrier and the associated barrier dysfunction have
An immune cell of the central This Review summarizes our current understand- become feasible because of the availability of an in vitro
nervous system that is derived
from mesodermal precursor
ing of the mechanisms that are involved in traversal of human brain microvascular endothelial cell (HBMEC)
cells and could be of the blood–brain barrier by selected meningitis-causing model 8–10,15,16 and in vivo models of experimental
haematopoietic lineage. microorganisms and the associated blood–brain barrier haematogenous meningitis in infant rats and mice6,7,17–19.
dysfunction.
Division of Pediatric Infectious
Diseases, Johns Hopkins Microbial traversal of the blood–brain barrier
University School of Medicine, The blood–brain barrier Pathogens can cross the blood–brain barrier transcellu-
200 North Wolfe Street, The blood–brain barrier (FIG. 1) is a structural and larly, paracellularly and/or by the Trojan-horse mecha-
Room 3157, Baltimore, functional barrier that is formed by brain microv- nism (FIG. 2). Transcellular traversal refers to microbial
Maryland 21287, USA.
e‑mail: kwangkim@jhmi.edu
ascular endothelial cells, astrocytes and pericytes. It penetration through barrier cells without any evidence
doi:10.1038/nrmicro1952 maintains the neural microenvironment by regulating of microorganisms between the cells or of intercellu-
Published online 7 July 2008 the passage of molecules into and out of the brain, and lar tight-junction disruption. Paracellular traversal is
nature reviews | microbiology volume 6 | august 2008 | 625
© 2008 Macmillan Publishers Limited. All rights reserved.
REVIEWS
Bacterial invasion and traversal
Cerebrospinal fluid Several studies in humans and experimental animals
point to a relationship between the magnitude of bacter-
aemia and the development of meningitis following
Neuron
E. coli, S. agalactiae and S. pneumoniae infections17,33,35–39.
Other routes of bacterial entry into the CNS include spread
from a contiguous source of infection, such as sinusitis
or mastoiditis. For example, S. pneumoniae has been
Astrocyte shown to enter the CNS through a non-haematogenous
Microglial cell
route in experimental animals after intranasal inocula-
tion and otitis media39,40. E. coli penetration into the CNS
is similar in infant and adult animals with similar levels
Pericyte of bacteraemia17, which suggests that a threshold level
of bacteraemia must be reached before the blood–brain
barrier can be breached.
The prevention of bacterial multiplication in the blood
could therefore be a potential approach for the pre-
Blood vessel Endothelial cell vention of bacterial meningitis. E. coli K1 capsular
polysaccharides and O‑chain lipopolysaccharides have
been recognized as crucial surface structures that con-
Figure 1 | The blood–brain barrier. The blood–brain barrier is formed by brain tribute to resistance to serum- and polymorphonuclear
microvascular endothelial cells, astrocytes and pericytes. It maintains the neural
Nature Reviews | Microbiology leukocyte-mediated killing17,41. Such resistance is a
microenvironment by regulating the passage of molecules into and out of the brain, and factor in the induction of a high degree of bacteraemia,
protects the brain from any microorganisms and toxins that are circulating in the blood. and studies are underway to identify additional microbial
structures that contribute to a high level of bacteraemia
and that could be used as protective epitopes in vaccine
defined as microbial penetration between barrier cells design42. This concept has been successfully applied to
with and/or without evidence of tight-junction disrup- Haemophilus influenzae serotype b and S. pneumoniae,
tion. The Trojan-horse mechanism involves microbial and protein-conjugated capsular polysaccharide vaccines
penetration of the barrier cells using transmigration for these organisms have almost completely eliminated
within infected phagocytes. the meningitis that is caused by the vaccine serotypes.
Transcellular traversal of the blood–brain barrier For example, introduction of the 7-valent pneumococcal
has been demonstrated for most meningitis-causing conjugate vaccine (PCV7) led to a substantial reduction
bacterial pathogens, including E. coli6,7,17, Streptococcus in the incidence of pneumococcal meningitis that was
agalactiae 6,7,20, S. pneumoniae 6,7,21, Neisseria menin- caused by PCV7 serotypes in the target population of
gitidis 6,7,22 and fungal pathogens, such as Candida children aged less than 5 years43,44. Use of the vaccine also
albicans 23 and Cryptococcus neoformans 24 (BOX 1) . reduced pneumococcal meningitis among unvaccinated
Paracellular penetration of the blood–brain barrier has populations through reductions in nasopharyngeal colo-
been suggested for the protozoan Trypanosoma spp.25,26 nization and transmission of vaccine-type pneumococci
and Borrelia spp.27,28, although these microorganisms from vaccinated children43,44. One caveat is the appar-
have also been shown to traverse the blood–brain ent increase in the incidence of invasive pneumococcal
barrier by transcellular penetration25,27,28. The Trojan- disease that is caused by non-PCV7 serotypes, such as
horse mechanism has been suggested for Listeria serotype 19A45. By contrast, it is unclear whether the
monocytogenes and Mycobacterium tuberculosis29, but development of meningococcal (N. meningitidis) men-
transcellular penetration of the blood–brain barrier has ingitis is associated with a threshold level of bacteraemia,
also been demonstrated for these organisms6,7,30–32. although prevention of invasive meningococcal disease
The availability of in vivo models has enabled us to has been achieved by the use of meningococcal group C
elucidate some of the mechanisms that are involved conjugate vaccines46.
in the traversal of the blood–brain barrier by micro- Recent studies have shown that a high degree of
organisms. For example, studies have indicated that bacteraemia is necessary, but not sufficient, for the devel-
the primary site of entry into the CNS for circulating opment of meningitis and that microbial binding to, and
E. coli, S. agalactiae and C. neoformans is the cerebral invasion of, HBMECs is a prerequisite for penetration of
vasculature, not the choroid plexus17,24,33,34. The interac- the blood–brain barrier in vivo. This was demonstrated
tion between E. coli and HBMECs is currently the best- using infant rats with experimental haematogenous E. coli
characterized system to study how meningitis-causing meningitis that had been infected with several isogenic
Choroid plexus pathogens cross the blood–brain barrier6,7. In the fol- mutants of E. coli K1 strain RS218 in which proteins that
A site of production of lowing sections, the mechanisms that are involved in contribute to HBMEC binding (such as outer-membrane
cerebrospinal fluid in the adult
traversal of the blood–brain barrier by microorgan- protein A (OmpA)) and invasion (such as IbeA, IbeB,
brain that is formed by the
invagination of ependymal cells
isms that commonly cause CNS infections in humans IbeC and cytotoxic necrotizing factor 1 (CNF1)) had
into the ventricles, which then are summarized, with an emphasis on the similarities been deleted. The deleted strains were significantly less
become vascularized. and differences between different organisms. able to traverse the blood–brain barrier than the parental
626 | august 2008 | volume 6 www.nature.com/reviews/micro
© 2008 Macmillan Publishers Limited. All rights reserved.
REVIEWS
a Transcellular No free bacteria are found in the cytoplasm of HBMECs
traversal Microorganism or between adjacent HBMECs, which confirms that
E. coli K1 and S. agalactiae cross the blood–brain barrier
Blood
Endothelial cell by transcellular penetration.
Pathogenic microorganisms use various strategies to
invade host cells, such as endothelial cells. Two of the
major mechanisms involve rearrangements of the host
cell actin cytoskeleton: the zipper mechanism, which
Brain
involves the formation of cell protrusions that contact
the pathogen, and the trigger mechanism, which involves
the formation of membrane ruffles around the patho-
gen53,54. Invasion of HBMECs by meningitis-causing
b Paracellular microorganisms also requires rearrangement of the actin
traversal
cytoskeleton6,7,20,30,52. However, the mechanisms that are
involved differ between microorganisms6,7,16,55–57. Once
internalized into membrane-bound vacuoles6,20,52,57,58,
some bacteria can modulate intracellular trafficking to
avoid lysosomal fusion.
In vivo, HBMECs are exposed to shear stress through
blood flow, which enhances the barrier properties of a
HBMEC monolayer59. N. meningitidis adhesion to brain
endothelial cells is inversely affected by shear stress60,
c Trojan-horse mechanism which suggests that cerebral microcirculation is an
important attribute in N. meningitidis adhesion to the
Macrophage blood–brain barrier.
It is important to note that E. coli K1 binding to, and
invasion of, endothelial cells has been demonstrated in
cells that are derived from the brain, such as HBMECs,
but not in cells of non-brain origin, such as human
umbilical vein endothelial cells (HUVECs), human aortic
arterial endothelial cells and human iliac vein endothe-
lial cells61,62, which suggests that the interaction between
meningitis-causing E. coli K1 and HBMECs is unique.
Similarly, Streptococcus suis (a causative agent of men-
ingitis and septicaemia) has been shown to interact with
HBMECs but not HUVECs63. It is therefore unclear
whether the interactions between meningitis-causing
microorganisms and non-brain endothelial cells, such
Figure 2 | Mechanisms involved inReviews
Nature microbial traversal
| Microbiology as bone-marrow-derived endothelial cells64, can be
of the blood–brain barrier. Pathogens can cross the extrapolated to interactions with HBMECs.
blood–brain barrier transcellularly, paracellularly and/or
in infected phagocytes (the Trojan-horse mechanism).
Bacterial ligand–receptor interactions
a | In transcellular traversal, the pathogens cross the
barrier without any evidence of intercellular tight- E. coli. As discussed above, microbial binding to and
junction disruption or detection of microorganisms invasion of HBMECs is a prerequisite for successful
between cells. b | Paracellular traversal involves penetration into the CNS6,7. Type 1 fimbriae and OmpA
microbial penetration between barrier cells with are the two major determinants that contribute to E. coli
and/or without evidence of tight-junction disruption. K1 binding to HBMECs65,66. FimH (a type 1 fimbrial
c | The Trojan-horse mechanism involves microbial adhesin) interacts with CD48 on HBMECs, and the
penetration of the barrier cells using transmigration addition of exogenous FimH- or CD48-specific anti-
within infected phagocytes. bodies inhibits E. coli K1 binding to HBMECs67. It has
also been shown that the amino‑terminal region and
strain despite causing similar levels of bacteraemia (FIG. 3), surface-exposed loops of OmpA contribute to binding
which indicates that those E. coli proteins that contribute to HBMECs68 and that OmpA interacts with HBMECs
to HBMEC binding and invasion are necessary for through N‑acetylglucosamine (GlcNAc) residues in
crossing the blood–brain barrier in vivo6,7,47–51. HBMEC glycoproteins, including gp96 (Refs 66,69).
Transmission electron microscopy studies have Exogenous OmpA and gp96 (also known as GRP94)
revealed that E. coli K1 and S. agalactiae invade HBMECs. and anti-gp96 antibodies inhibit E. coli K1 binding
Internalized bacteria are found within membrane-bound to HBMECs, but do not inhibit binding of an OmpA
vacuoles and transmigrate HBMECs in an enclosed mutant66,68. The ability of the OmpA mutant to penetrate
vacuole without intracellular multiplication and without into the CNS was significantly reduced compared with
any change in the integrity of HBMEC monolayers20,52. the parental E. coli K1 strain, and GlcNAc‑β1,4-GlcNAc
nature reviews | microbiology volume 6 | august 2008 | 627
© 2008 Macmillan Publishers Limited. All rights reserved.
REVIEWS
Box 1 | Cryptococcal invasion and traversal of the blood–brain barrier
Cryptococcus neoformans is a common cause of culture-proven meningitis in areas of the world where HIV‑1 is endemic, and
cryptococcal meningitis is known for its high morbidity and mortality rates118. C. neoformans is commonly acquired by
inhalation. Extrapulmonary dissemination can lead to infection of the bloodstream and subsequent haematogenous
dissemination to target organs, most commonly resulting in meningoencephalitis.
Results from experimental mouse models of cryptococcal meningitis following intravenous inoculation, as well as cases of
human cryptococcal meningitis, have indicated that C. neoformans invasion into the brain follows fungaemia, and that
cerebral capillaries, not the choroid plexus, are the portals of entry into the brain24,119,120. Cryptococcal invasion of the brain
does not require recruitment of host inflammatory cells24,119,120, which eliminates the possibility that C. neoformans traverses
the blood–brain barrier using the Trojan-horse mechanism.
A recent study showed that C. neoformans strains can enter and traverse human brain microvascular endothelial cells
(HBMECs) without any obvious change in HBMEC integrity. Transmission and scanning electron microscopy has revealed
that C. neoformans induces the formation of microvilli-like protrusions to initiate entry into HBMECs, that C. neoformans is
found intracellularly in membrane-bound vacuoles and that no free C. neoformans cells are found in the HBMEC cytoplasm24.
These findings indicate that C. neoformans uses a transcellular mechanism (FIG. 2a) to enter HBMECs that involves host cell
actin cytoskeleton rearrangements.
Several studies have shown that various cryptococcal virulence factors contribute to extrapulmonary dissemination,
including the capsular polysaccharide, mannitol, the mating type, melanin, phenotypic switching, phospholipase,
prostaglandins and urease118 (TABLE 1). For example, mutants of C. neoformans that lacked laccase were defective in
dissemination to extrapulmonary sites in mice following intratracheal inoculation compared with the parental strain, but
seemed to be as effective as the parental strain in dissemination to the brain following intravenous inoculation121.
Similarly, mutants of C. neoformans that lacked phospholipase B were defective in extrapulmonary dissemination
following intratracheal inoculation compared with the parental strain; the ability of these mutants to disseminate to the
brain following intravenous inoculation is unknown122,123. A recent report described how cryptococcal urease contributes
to dissemination to the central nervous system (CNS) following intratracheal and intravenous inoculation120, but it is
unclear how urease contributes to C. neoformans traversal of the blood–brain barrier. The cryptococcal inositolphosphos-
phingolipid phospholipase C1 gene (isc1) has been shown to be important for controlling the dissemination of
C. neoformans to the brain in mice in which macrophages are depleted124. This suggests that macrophage activation is
important for preventing fungal dissemination of the ∆isc1 mutant to the CNS and the development of C. neoformans
meningoencephalitis. Additional studies are therefore needed to elucidate the microbial–host interactions and associated
signal-transduction pathways that are involved in C. neoformans traversal of HBMECs and penetration into the CNS.
oligomers blocked E. coli K1 traversal of the blood–brain the fact that a polyclonal antibody raised against this recep-
barrier in the infant-rat model of experimental haema- tor inhibited E. coli K1 invasion of HBMECs74. In addi-
togenous meningitis51,62. These findings indicate that tion, enrichment of IbeA-receptor-expressing HBMECs
proteins such as FimH and OmpA contribute to the bind- by fluorescence-activated cell sorting resulted in sig-
ing of E. coli to HBMECs through an interaction with nificantly enhanced invasion by the IbeA-positive E. coli
their respective HBMEC receptors (TABLE 1). gp96 is an K1 strain74. CNF1 is a virulence factor that is associated
endoplasmic reticulum paralogue of heat shock protein with pathogenic E. coli strains that cause extraintestinal
90 that is not restricted to the endoplasmic reticulum and infections, including meningitis. CNF1 is an AB‑type
has been detected at the surface of HBMECs66. gp96 is toxin, is composed of an amino‑terminal cell-binding
also a cellular receptor for L. monocytogenes Vip, which domain and a carboxy‑terminal catalytic domain that
is involved in infection of the spleen, liver and brain of has deaminase activity, and activates Rho GTPases, such
mice70; however, gp96 interactions with OmpA and Vip as Rho, Rac and Cdc42 (Refs 75,76). CNF1 contributes to
involve different signal-transduction pathways70. E. coli K1 invasion of HBMECs in vitro and traversal of the
A recent study used an E. coli DNA microarray to blood–brain barrier in vivo, and these in vitro and in vivo
compare an OmpA mutant with its parental E. coli K1 effects are dependent on RhoA activation49. However, it
strain and revealed that the OmpA mutant expressed sig- is unclear how CNF1 enters HBMECs and activates Rho
nificantly fewer fim cluster genes71. This suggested that the GTPases. It has been suggested that CNF1 is internalized
decreased binding of the OmpA mutant might be related by receptor-mediated endocytosis upon binding to a cell-
to lower expression of type 1 fimbriae. Additional studies surface receptor77. The HBMEC receptor for CNF1 has
are needed to determine whether the in vitro and in vivo been identified as a 37-kDa laminin receptor precursor
defects of the OmpA mutant are related to the decreased (37 LRP)78. The 37 LRP is a ribosome-associated cyto-
expression of type 1 fimbriae and to understand how the plasmic protein and is a precursor of the 67-kDa laminin
deletion of ompA affects type 1 fimbriae expression. receptor (67 LR). It is unclear how the mature 67 LR is
AB-type toxin
A bacterial toxin that modifies As mentioned earlier, several other E. coli determi- synthesized from the 37 LRP, but mature 67 LR is present
target proteins within the nants that contribute to invasion of HBMECs have been on the cell surface and functions as a membrane receptor
cytosol of host cells and is identified47–50,72. Recombinant Ibe proteins inhibit E. coli for the adhesive basement-membrane protein laminin79.
composed of two domains: one K1 invasion of HBMECs47,73, which suggests that these It has been shown that the expression levels of 37 LRP
that is responsible for the
enzymatic activity (A) and one
proteins also contribute to HBMEC invasion through and 67 LR are directly correlated with CNF1-mediated
that is responsible for cell- ligand–receptor interactions. This was supported by the E. coli K1 invasion of HBMECs78. Recent studies have
receptor binding (B). identification of a HBMEC receptor protein for IbeA and shown that CNF1-expressing E. coli K1 upregulates the
628 | august 2008 | volume 6 www.nature.com/reviews/micro
© 2008 Macmillan Publishers Limited. All rights reserved.
REVIEWS
Bacteraemia Meningitis penetration into the CNS. gC1q‑R has also been shown to be
10 120 the HBMEC receptor for Plasmodium falciparum-infected
red blood cells (Pf-IRBCs)92.
Bacteraemia (log CFU per ml blood)
9
100
8
S. pneumoniae. S. pneumoniae crosses the blood–brain
7
Meningitis (%)
80 barrier partly through interactions between cell-wall
6
phosphorylcholine and the platelet-activating-factor
5 60 (PAF) receptor. This was shown by its partial inhibition of
4 pneumococcal invasion of HBMECs by a PAF-receptor
40
3 antagonist21,93 and delayed translocation of pneumococci
2
20
from the lung to the blood (as well as from the blood to
1 the cerebrospinal fluid) in PAF-receptor-knockout mice94.
0 0 The PAF receptor has also been shown to interact with
H. influenzae serotype b95, but the role of the PAF recep-
RS218
∆ompA
RS218
∆ibeA
RS218
∆ibeB
RS218
∆ibeC
RS218
∆aslA
RS218
∆cnf1
tor in H. influenzae penetration into the CNS remains
Figure 3 | The effect of microbial determinants
Nature on
Reviews | Microbiology
unclear. Several studies suggested that the role of the PAF
Escherichia coli meningitis. In infant rats with experimental receptor differs in S. pneumoniae and H. influenzae infec-
haematogenous E. coli meningitis, isogenic mutants of E. coli tions. For example, a PAF-receptor antagonist attenuated
K1 strain RS218 in which outer-membrane protein A the pleocytosis that was elicited by intracisternal inocula-
(OmpA), IbeA, IbeB, IbeC, arylsulfatase-like protein (AslA) tion of S. pneumoniae but had no effect on the pleocytosis
and cytotoxic necrotizing factor 1 (CNF1) had been deleted that was induced by H. influenzae96.
were less able to traverse the blood–brain barrier and cause
meningitis than the parental strain, despite causing similar N. meningitidis. N. meningitidis is a human-specific
levels of bacteraemia. CFU, colony-forming unit.
pathogen that interacts with human endothelial and
epithelial cells. This interaction involves several micro-
expression of 67 LR in HBMECs and recruits 67 LR along bial structures and proteins, including type IV pili,
with focal adhesion kinase (FAK) and paxillin to the site PilC, N. meningitidis adhesin A (NadA) and the Opa
of invading E. coli K1 in a CNF1-dependent manner80. and Opc proteins64,97–99. However, these observations
The 37 LRP–67 LR has been shown to be a cellular target were derived from N. meningitidis interactions with
for a range of CNS-infecting microorganisms, including non-brain endothelial cells, such as human bone-
dengue virus, adeno-associated virus and Venezuelan marrow-derived endothelial cells, and their relevance to
equine encephalitis virus, as well as the prion protein HBMECs remains unclear. Interestingly, the invasion
PrP81–84. It remains to be determined how these different of HBMECs by unencapsulated N. meningitidis is medi-
organisms interact with the same receptor. ated by Opc binding to fibronectin, which anchors the
bacteria to the integrin α5β1 receptor on the HBMEC
S. agalactiae and L. monocytogenes. Recent studies surface22. However, in the bloodstream, N. meningitidis
have indicated that other meningeal pathogens invade is encapsulated, and the in vivo relevance of Opc–
HBMECs through ligand–receptor interactions (TABLE 1). fibronectin-mediated binding to integrin is therefore
The neonatal meningitis pathogens S. agalactiae and unclear. N. meningitidis pili bind to CD46 on HBMECs98,
L. monocytogenes possess several proteins that allow and their lipooligosaccharides have been shown to con-
binding to, and invasion of, HBMECs. S. agalactiae tribute to a high level of bacteraemia and subsequent
binding to HBMECs occurs through laminin-binding penetration into the CNS100; CD46 has also been shown
protein (Lmb), the fibrinogen-binding protein FbsA, to be the receptor for measles virus, adenovirus and
pili and invasion-associated gene A (IagA) (through human herpesvirus 6 (Refs 101–103).
lipoteichoic acid anchoring)85–88, but it is unclear whether
these proteins are unique to cerebrospinal fluid isolates of M. tuberculosis. Tuberculosis of the CNS is a serious and
S. agalactiae and whether they contribute to S. agalactiae often fatal disease that affects young children dispropor-
penetration into the CNS. tionally. M. tuberculosis can cross the blood–brain barrier
L. monocytogenes invasion of HBMECs is mediated by as a free organism or in infected phagocytes104 (FIG. 2). One
internalin B (InlB)30. Several HBMEC receptors for InlB recent study showed that strains of M. tuberculosis can
have been identified, including gC1q‑R (the receptor for invade and traverse HBMECs; invasion was significantly
the globular head of the complement component C1q) increased for the M. tuberculosis strains H37RV and CDC
and Met tyrosine kinase89,90, but their contributions to 1551 than for the non-pathogenic Mycobacterium smeg-
L. monocytogenes invasion of HBMECs are not com- matis, and traversal occurred with M. tuberculosis strains
pletely understood. For example, InlB does not compete but not M. smegmatis31.
for the same interaction site on Met as the natural ligand DNA microarray analysis identified at least 33
hepatocyte growth factor91. L. monocytogenes penetration genes that were upregulated by eightfold or more and
into the CNS has also been attributed to transmigration of 147 genes that were downregulated by eightfold or more
Pleocytosis
The presence of a higher than L. monocytogenes-infected monocytes and myeloid cells in M. tuberculosis that was associated with HBMECs
normal number of cells in the across the blood–brain barrier29, and further studies are compared with M. tuberculosis that was not associated
cerebrospinal fluid. needed to determine the major route of L. monocytogenes with HBMECs31. Mutants of five M. tuberculosis genes
nature reviews | microbiology volume 6 | august 2008 | 629
© 2008 Macmillan Publishers Limited. All rights reserved.
REVIEWS
Table 1 | Ligand–receptor interactions in microbial traversal of the blood–brain barrier
Ligand Receptor and mechanism Refs
Escherichia coli
FimH CD48; binding to HBMECs 65,67
OmpA gp96; binding to HBMECs 6,68,69
CNF1 37 LRP–67 LR; invasion of HBMECs 49,78,80
IbeA 45 kDa protein; invasion of HBMECs 47,74
Listeria monocytogenes
InlB gClq or Met; invasion of HBMECs 30,89,90
Vip gp96; penetration into the brain 70
Neisseria meningitidis
Opc Fibronectin; invasion of HBMECs through α5β1 integrin 22,97,108
Pili Possibly CD46; penetration into the brain 97,98
LOS Unknown; penetration into the brain owing to a high level of bacteraemia 100
Streptococcus pneumoniae
Phosphorylcholine PAF receptor; invasion of HBMECs 21,93
Haemophilus influenzae serotype b
Phosphorylcholine PAF receptor; unknown 95
Streptococcus agalactiae
Lmb Laminin; invasion of HBMECs 85
FbsA Fibrinogen; binding to HBMECs 86
Pili (PilA and PilB) Unknown; binding to HBMECs 88
iagA Unknown; binding to HBMECs through LTA anchoring 87
Cryptococcus neoformans
Capsule, laccase, phospholipase Unknown; penetration into the brain 24, 118–124
B, urease and Isc1
Plasmodium falciparum
Pf-IRBCs or PfEMP1 Thrombospondin, CD36, ICAM1, gClqR, PECAM or CD31, VCAM1, ELAM1 92,113–116
and chondroitin sulphate A; HBMEC dysfunction
37 LRP–67 LR, 37-kDa laminin receptor precursor–67-kDa laminin receptor; CNF1, cytotoxic necrotizing factor 1; ELAM1,
endothelial-leukocyte adhesion molecule 1; gp96, glycoprotein 96; HBMEC, human brain microvascular endothelial cell; iagA,
invasion-associated gene A; ICAM1, intercellular adhesion molecule 1; InlB, internalin B; Isc1, inositolphosphosphingolipid
phospholipase C1; Lmb, laminin-binding protein; LOS, lipooligosaccharide; LTA, lipoteichoic acid; OmpA, outer-membrane protein A;
Pf-IRBC, P. falciparum-infected red blood cell; PfEMP1, P. falciparum erythrocyte membrane protein 1; PAF, platelet-activating
factor; PECAM, platelet–endothelial-cell adhesion molecule.
were attenuated in their ability to invade and/or survive — might lead to focal and transient degradation of tight-
in the brain32. The identification and characterization of junction proteins, which would allow B. burgdorferi to
M. tuberculosis genes that are involved in traversal of the traverse the blood–brain barrier. Other recent studies
blood–brain barrier should help elucidate the pathogenesis revealed that Borrelia turicatae serotype 1, which is
of CNS tuberculosis. defined by the presence of Vsp1 (variable small protein
1), infects the CNS significantly more than the isogenic
Spirochaetes. Neurosyphilis and neuroborreliosis are serotype, which is defined by the presence of Vsp2, and
prototypic spirochaete infections of the CNS, but the that Vsp1 binds to HBMECs28. B. burgdorferi expressing
mechanisms that are involved in their traversal of recombinant Vsp1 exhibited an increased interaction with
the blood–brain barrier remain unclear. Treponema pal- HBMECs. These findings suggest that Vsp1 binding to
lidum can invade through the intercellular junction of aor- HBMECs might play a part in borrelial penetration into
tic endothelial cells105, which suggests that a mechanism the CNS.
of paracellular penetration of the vascular endothelium
Fibrinolytic system occurs, but it is unclear whether a similar mechanism is Receptors and cytokines. Cytokines have been shown
A broad spectrum of involved in T. pallidum penetration of the blood–brain to regulate expression of the HBMEC receptors and/or
proteolytic enzymes that barrier. A previous study showed that Borrelia burgdor- signalling molecules that are involved in the interaction
includes the plasminogen feri strains traverse HBMECs without any obvious change between HBMECs and microorganisms. For example,
activator system and plasmin.
Plasmin and plasmin activators
in the integrity of HBMECs and that this traversal is tumour necrosis factor (TNF)‑α treatment of HBMECs
proteolytically degrade the facilitated by proteases27. These findings suggest that the resulted in increased invasion of S. pneumoniae owing to
extracellular matrix. fibrinolytic system — linked by an activation cascade upregulation of the PAF receptor21, which is the receptor
630 | august 2008 | volume 6 www.nature.com/reviews/micro
© 2008 Macmillan Publishers Limited. All rights reserved.
REVIEWS
E. coli K1 E. coli K1, S. agalactiae and L. monocytogenes. E. coli K1
invasion and traversal of the blood–brain barrier involves
FAK, paxillin, phosphatidylinositol 3‑kinase, Src kinase,
Rho GTPases, cPLA2α and 5‑lipoxygenase6,7,49,55,56,66,76,80
FimH OmpA Ibe CNF1 (FIG. 4). By contrast, S. agalactiae invasion of HBMECs
CD48 BMEC gp96 R LR is independent of Src activation, and L. monocytogenes
invasion of HBMECs is independent of FAK and cPLA2
PIP3 PIP2 activation. The microbial–host interactions that contrib-
PH
ute to invasion of HBMECs and the relevant signalling
cPLA2α mechanisms that are involved have not yet been fully
Akt elucidated.
5-LO
It is important to note that the mechanisms which
p110
p85 P FAK are involved in entry of HBMECs by meningitis-causing
397 microorganisms differ from those that are involved in the
Rho GTPases release of cytokines and chemokines in response to these
Rho GTPases
microorganisms. For example, E. coli proteins that are
Cytoskeleton
rearrangement involved in binding to and invasion of HBMECs (OmpA
and CNF1, respectively) did not affect the release of inter-
leukin-8 (IL‑8) from HBMECs107. Similar findings were
E. coli K1 invasion
observed for an S. agalactiae Lmb mutant, which was
defective for the invasion of HBMECs but induced the
Figure 4 | Signalling mechanisms involved in EscherichiaNature coli K1-mediated actin
Reviews | Microbiology same amount of IL‑8 as the parental strain85. Additionally,
cytoskeleton rearrangements and traversal of the blood–brain barrier. E. coli K1 N. meningitidis invasion of HBMECs involves c‑Jun kinases
invasion and traversal of the blood–brain barrier occurs as the result of specific bacterial 1 and 2 (JNK1 and JNK2), but the release of IL‑6 and
interactions with receptors (CD48, gp96, R and LR) on brain microvascular endothelial IL‑8 from HBMECs involves the p38 mitogen-activated
cells (BMECs) and involves FAK, paxillin, phosphatidylinositol 3-kinase, Rho GTPases, protein kinase (MAPK) pathway108.
5-LO and cPLA2α. 5-LO, 5-lipoxygenase; CNF1, cytotoxic necrotizing factor 1; cPLA2α,
Another crucial factor for the development of meningi-
cytosolic phospholipase A2α; FAK, focal adhesion kinase; gp96, glycoprotein 96; OmpA,
outer-membrane protein A; PH, pleckstrin homology; PIP, phosphatidylinositol phosphate. tis is the ability of pathogens to cross the blood–brain bar-
rier as live organisms. As discussed earlier, transmission
electron microscopy studies with E. coli and S. agalactiae
for S. pneumoniae phosphorylcholine. Transforming (as well as C. neoformans, C. albicans and M. tuberculosis)
growth factor (TGF)‑β1 treatment of HBMECs sig- revealed that these microorganisms are found within
nificantly increased E. coli K1 invasion and traversal of membrane-bound vacuoles in HBMECs and transmigrate
HBMECs106, whereas treatment with TNF‑α and inter- HBMECs in an enclosed vacuole. HBMECs possess the
feron (IFN)‑γ had no effect. TGF‑β1 has been shown to complete trafficking machinery that is necessary to deliver
affect the host cell signal-transduction pathways that are microorganism-containing vacuoles to lysosomes 57.
involved in microbial invasion and traversal of HBMECs, Vacuoles that contained a capsule-deletion mutant of
such as Rho GTPases and cytosolic phospholipase A2α E. coli K1 interacted sequentially with early endosomal
(cPLA2α), and more work is needed to understand the marker proteins (such as early endosomal auto-antigen 1
mechanisms that are associated with cytokine-mediated and the transferrin receptor) and late endosome and late
modulation of microbial–HBMEC interactions. endosome–lysosomal markers (such as Rab7 and lyso-
Further studies are needed to elucidate the some-associated membrane proteins, respectively), and
ligand–receptor interactions that are involved in micro- allowed lysosomal fusion with subsequent degradation
bial traversal of the blood–brain barrier, particularly as inside vacuoles. By contrast, capsule-positive E. coli K1-
the same receptors have been shown to be involved in the containing vacuoles reached early and late endosomes
pathogenesis of CNS infection by different microorgan- without fusion with lysosomes, thereby allowing E. coli
isms (TABLE 1). It remains speculative whether the expres- K1 to cross the blood–brain barrier as live bacteria57.
sion levels of these receptors dictate the tropism for CNS These findings indicate that the capsule of E. coli K1
infection by meningitis-causing microorganisms. modulates the maturation of E. coli K1-containing
vacuoles and prevents fusion with lysosomes, which is
Signal-transduction mechanisms necessary for traversal of the blood–brain barrier as live
Recent advances in our understanding of eukaryotic signal- bacteria. Additional studies are needed to understand
transduction pathways have expedited our understanding how the capsule can modulate intracellular vacuolar
of microbial invasion and traversal of the blood–brain trafficking and whether similar events occur with other
barrier, including identification of the signalling mol- meningitis-causing microorganisms.
ecules that are involved in rearrangements of the host cell
actin cytoskeleton. The signal-transduction mechanisms Consequences of CNS infection
that are involved in actin cytoskeleton rearrangements As discussed above, CNS-infecting microorganisms
and HBMEC invasion differ between meningitis-causing can induce blood–brain barrier dysfunction by affect-
microorganisms. For example, actin cytoskeleton rear- ing the release and/or expression of cytokines, chem-
rangements are a prerequisite for HBMEC invasion by okines and cell-adhesion molecules and/or inducing
nature reviews | microbiology volume 6 | august 2008 | 631
© 2008 Macmillan Publishers Limited. All rights reserved.
REVIEWS
Box 2 | Parasite invasion and traversal of the blood–brain barrier invade HBMECs85,107. In addition, the signalling pathways
that are involved in the release of IL‑8 differed from the
The neurological manifestations of sleeping sickness in humans that are caused by pathways that are involved in the invasion of HBMECs
Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense are attributed by N. meningitidis (p38 MAPK and JNK, respectively)108.
to the penetration of the central nervous system by these organisms25,26.
Notably, the release of IL‑8 in response to meningitis-
Bloodstream forms of T. brucei gambiense efficiently cross human brain
causing microorganisms did not occur in non-brain endothe-
microvascular endothelial cells (HBMECs) by a paracellular route26. In experimental
rodent models, the parasite can pass through the blood–brain barrier across or lial cells such as HUVECs107,112,113, which illustrates how
between endothelial cells and the vessel basement membranes25. The laminin the IL‑8 response to meningitis-causing microorganisms
composition of the basement membranes determines whether they are permissive is specific to HBMECs.
to parasite penetration, and interferon (IFN)‑γ has been shown to have an important Another consequence of the microbial interaction with
role in regulating trypanosome trafficking into the brain25. HBMECs is encephalopathy, which has been observed in
Toxoplasma encephalitis is a serious complication of infection with the obligate patients with cerebral malaria and B. pertussis infection.
intracellular parasite Toxoplasma gondii. Stimulation of HBMECs with IFN‑γ resulted in The mechanisms that are involved in infection-related
the restricted growth of T. gondii, which was enhanced in the presence of tumour necrosis encephalopathy remain incompletely understood. For
factor (TNF)‑α. This anti-parasitic activity was due to the induction of indoleamine
example, Pf-IRBCs have been shown to bind to several
2,3‑dioxygenase (IDO) in HBMECs by IFN‑γ and TNF-α13. Repletion of the essential amino
molecules on HBMECs92,114–116 (TABLE 1) and the binding
acid tryptophan abrogated this inhibition, suggesting that IDO activation and the
subsequent degradation of tryptophan is the main mechanism for IFN‑γ-mediated and of Pf-IRBCs to HBMECs and/or the sequestration of
TNF-α-mediated toxoplasmosis. IDO-positive HBMECs can cleave tryptophan to Pf-IRBCs in the cerebral microvasculature has been shown
kynurenine and therefore reduce the transport of tryptophan to astrocytes. As IDO is the to cause blood–brain barrier dysfunction, including the
main effector mechanism in astrocytes against T. gondii, reduced tryptophan influx release of cytokines and chemokines, increased expression
enhances the antimicrobial effect of IDO-positive astrocytes. Additional studies are of cell-adhesion molecules, alterations in intercellular pro-
needed to elucidate the mechanisms that are involved in IDO-mediated toxoplasmosis. teins and the induction of apoptosis114–116. Encephalopathy
is a serious complication of B. pertussis infection, and a
cytotoxicity and apoptosis in HBMECs, which results recent study has shown that pertussis toxin induces
Encephalopathy
Brain dysfunction that is in increased blood–brain barrier permeability, pleo- a transient increase in permeability and transendothelial
associated with alterations of cytosis and encephalopathy6. For example, blockade of migration of monocytes in HBMEC monolayers117, which
the neural microenvironment pleocytosis by intravenous administration of an anti- suggests that pertussis-toxin-mediated blood–brain
and results from metabolic, CD18 monoclonal antibody that was directed against dysfunction could contribute to encephalopathy.
toxic, vascular, infectious
and/or inflammatory insults.
the β‑chain of β2-integrin reduced blood–brain barrier
permeability in experimental meningitis that involved Conclusions
intracisternal injection of S. pneumoniae, N. meningitidis A major limitation to advances in the prevention and
and H. influenzae serotype b109. However, intracisternal treatment of CNS infection is our incomplete under-
injection of S. pneumoniae and H. influenzae serotype b standing of these diseases and the associated blood–brain
resulted in increased blood–brain barrier permeability barrier dysfunction. Studies of the in vitro model of the
in both normal animals and leukopenic animals110,111. blood–brain barrier and the in vivo animal model of
In addition, pleocytosis was not affected in intercellular experimental haematogenous meningitis have shed
adhesion molecule 1-knockout and E‑ and P‑selectin some light on the mechanisms of microbial traversal of
knockout mice with haematogenous meningitis caused the blood–brain barrier, the key step for the development
by S. pneumoniae and H. influenzae serotype b18,19. These of CNS infections. This traversal is the result of specific
findings indicate that increased blood–brain barrier per- microorganism–host interactions and involves host cell
meability and pleocytosis might develop independently of signal-transduction pathways that affect host cell actin
each other in bacterial meningitis. cytoskeleton rearrangements. It is unclear, however,
As noted above, meningitis-causing microorganisms whether the mechanisms that are involved in the traversal
such as E. coli, S. agalactiae, N. meningitidis and S. suis of the blood–brain barrier are similar and/or different in
induce the release of cytokines and chemokines from the various pathogens that cause CNS infection, such as
HBMECs, but the mechanisms that are involved differ bacteria, fungi and parasites (BOXes 1,2; TABLE 1). A more
from those which are involved in their binding to, and complete knowledge of microbial interactions with the
invasion of, HBMECs. For example, the release of IL‑8 in blood–brain barrier might facilitate the development of
response to E. coli and S. agalactiae infection was inde- novel strategies for the prevention and therapy of CNS
pendent of the ability of these organisms to bind to and infections.
1. World Health Organization. World health report: 6. Kim, K. S. Pathogenesis of bacterial meningitis: from cells contain tight and adherens junction proteins in
changing history (WHO, Geneva, 2004). bacteraemia to neuronal injury. Nature Rev. Neurosci. spatial proximity. Biochemistry 43, 5360–5369
2. Klinger, G., Chin, C.‑N., Beyene, J. & Perlman, M. 4, 376–385 (2003). (2004).
Predicting the outcome of neonatal bacterial A comprehensive review on the pathogenesis of 10. Stins, M. F., Badger, J. L. & Kim, K. S. Bacterial
meningitis. Pediatrics 106, 477–482 (2000). meningitis and the associated neuronal injury. invasion and transcytosis in transfected human brain
3. Stevens, J. P. et al. Long-term outcome of neonatal 7. Kim, K. S. Microbial translocation of the blood–brain microvascular endothelial cells. Microb. Pathog. 30,
meningitis. Arch. Dis. Child. Fetal Neonatal Ed. 88, barrier. Int. J. Parasitol. 36, 607–614 (2006). 19–28 (2001).
F179–F184 (2003). 8. Kim, Y. V., Di Cello, F., Hillaire, C. S. & Kim, K. S. 11. Rubin, L. L. & Staddon, J. M. The cell biology of the
4. Chang, C.-J. et al. Bacterial meningitis in infants: the Differential Ca2+ signaling by thrombin and protease- blood–brain barrier. Annu. Rev. Neurosci. 22, 11–28
epidemiology, clinical features, and prognostic factors. activated receptor-1-activating peptide in human (1999).
Brain Dev. 26, 168–175 (2004). brain microvascular endothelial cells. Am. J. Physiol. 12. Mucke, L. & Eddleston, M. Astrocytes in
5. van de Beek, D. et al. Clinical features and prognostic Cell Physiol. 286, C31–C42 (2004). infectious and immune-mediated diseases of the
factors in adults with bacterial meningitis. N. Engl. J. 9. Ruffer, C., Strey, A., Janning, A., Kim, K. S. & Gerke, V. central nervous system. FASEB J. 7, 1226–1232
Med. 351, 1849–1859 (2004). Cell–cell junctions of dermal microvascular endothelial (1993).
632 | august 2008 | volume 6 www.nature.com/reviews/micro
© 2008 Macmillan Publishers Limited. All rights reserved.
REVIEWS
13. Daubener, W. et al. Restriction of Toxoplasma gondii 33. Ferrieri, P., Burke, B. & Nelson, J. Production of 53. Knodler, L. A., Celli, J. & Finlay, B. B. Pathogenic
growth in human brain microvascular endothelial bacteremia and meningitis in infant rats with group B trickery: deception of host cell processes. Nature Rev.
cells by activation of indoleamine 2,3‑dioxygenase. streptococcal serotypes. Infect. Immun. 27, Mol. Cell Biol. 2, 578–588 (2001).
Infect. Immun. 69, 6527–6531 (2001). 1023–1032 (1980). 54. Cossart, P. & Sansonetti, P. J. Bacterial invasion: the
14. Anfuso, C. D. et al. Endothelial cell–pericyte 34. Berman, P. H. & Banker, B. Q. Neonatal meningitis. paradigms of enteroinvasive pathogens. Science
cocultures induce PLA2 protein expression through A clinical and pathological study of 29 cases. 304, 242–248 (2004).
activation of PKCα and MAPK/ERK cascade. J. Lipid Pediatrics 38, 6–24 (1966). 55. Reddy, M. A., Wass, C. A., Kim, K. S., Schlaepfer,
Res. 48, 782–793 (2007). 35. Kim, K. S. Effect of antimicrobial therapy for D. D. & Prasadarao, N. V. Involvement of focal
15. Stins, M. F., Gilles, F. & Kim, K. S. Selective experimental infections due to group B adhesion kinase in Escherichia coli invasion of
expression of adhesion molecules on human brain streptococcus on mortality and clearance of bacteria. human brain microvascular endothelial cells. Infect.
microvascular endothelial cells. J. Neuroimmunol. J. Infect. Dis. 155, 1233–1241 (1987). Immun. 68, 6423–6430 (2000).
76, 81–90 (1997). 36. Dietzman, D. E., Fischer, G. W. & Schoenknecht, 56. Reddy, M. A., Nemani, P. V., Wass, C. A. & Kim, K. S.
16. Das, A. et al. Differential role of cytosolic F. D. Neonatal Escherichia coli septicemia — Phosphatidylinositol 3‑kinase activation and interaction
phospholipase A2 in the invasion of brain bacterial counts in blood. J. Pediatr. 85, 128–130 with focal adhesion kinase in Escherichia coli K1
microvascular endothelial cells by Escherichia coli (1974). invasion of human brain microvascular endothelial cells.
and Listeria monocytogenes. J. Infect. Dis. 184, 37. Bell, L. M., Alpert, G., Campos, J. M. & Plotkin, J. Biol. Chem. 275, 36769–36774 (2000).
732–737 (2001). S. A. Routine quantitative blood cultures in children Showed for the first time that activation of FAK
Described the contribution of cPLA2 to E. coli with Haemophilus influenzae or Streptococcus and phosphatidylinositol 3-kinase is required for
invasion of brain microvascular endothelial cells. pneumoniae bacteremia. Pediatrics 76, 901–904 E. coli K1 invasion of HBMECs.
17. Kim, K. S. et al. The K1 capsule is the critical (1985). 57. Kim, K. J., Elliott, S. A., DiCello, F., Stins, M. F. &
determinant in the development of Escherichia coli 38. Sullivan, T. D., LaScolea, L. J. & Neter, E. Kim, K. S. The K1 capsule modulates trafficking of
meningitis in the rat. J. Clin. Invest. 90, 897–905 Relationship between the magnitude of bacteremia E. coli-containing vacuoles and enhances
(1992). in children and the clinical disease. Pediatrics 69, intracellular bacterial survival in human brain
18. Tan, T. Q., Smith, C. W., Hawkins, E. P., Mason, E. O. Jr 699–702 (1982). microvascular endothelial cells. Cell. Microbiol. 5,
& Kaplan, S. L. Hematogenous bacterial meningitis 39. Marra, A. & Brigham, M. D. Streptococcus 245–252 (2003).
in an intercellular adhesion molecule‑1‑deficient pneumoniae causes experimental meningitis First demonstration of the role of the K1 capsule
infant mouse model. J. Infect. Dis. 171, 342 (1995). following intranasal and otitis media infections via a in the modulation of E. coli movement in HBMECs.
19. Munoz, F. M., Hawkins, E. P., Bullard, D. C., nonhematogenous route. Infect. Immun. 69, 58. Nikulin, J., Panzner, U., Frosch, M. & Schubert-
Beauchet, A. L. & Kaplan, S. L. Host defense against 7318–7325 (2001). Unkmeir, A. Intracellular survival and replication of
systemic infection with Streptococcus pneumoniae is 40. van Ginkle, F. W. et al. Pneumococcal carriage Neisseria meningitidis in human brain microvascular
impaired in E‑, P‑, and E‑/P‑selectin‑deficient mice. results in ganglioside-mediated olfactory tissue endothelial cells. Int. J. Med. Microbiol. 296,
J. Clin. Invest. 100, 2099–2106 (1997). infection. Proc. Natl Acad. Sci. USA 24, 553–558 (2006).
20. Nizet, V. et al. Invasion of brain microvascular 14363–14367 (2003). 59. Siddharthan, V., Kim, Y. V., Liu, S. & Kim, K. S.
endothelial cells by group B streptococci. Infect. 41. Cross, A. S., Kim, K. S., Wright, D. C., Sadoff, J. C. & Human astrocytes/astrocyte conditioned medium
Immun. 65, 5074–5081 (1997). Gemski, P. Role of lipopolysaccharide and capsule in and shear stress enhance the barrier properties of
The first study to show transcellular penetration the serum resistance of bacteremic strains of human brain microvascular endothelial cells. Brain
of HBMECs by S. agalactiae. Escherichia coli. J. Infect. Dis. 154, 497–503 Res. 1147, 39–50 (2007).
21. Ring, A., Weiser, J. N. & Tuomanen, E. I. (1986). 60. Mairey, E. et al. Cerebral microcirculation shear
Pneumococcal trafficking across the blood–brain 42. Xie, Y. et al. Identification and characterization of stress levels determine Neisseria meningitidis
barrier. Molecular analysis of a novel bi-directional Escherichia coli RS218-derived islands in the attachment sites along the blood–brain barrier.
pathway. J. Clin. Invest. 102, 347–360 (1998). pathogenesis of E. coli meningitis. J. Infect. Dis. J. Exp. Med. 203, 1939–1950 (2006).
22. Unkmeir, A. et al. Fibronectin mediates Opc- 194, 358–364 (2006). 61. Stins, M. F. et al. Binding characteristics of
dependent internalization of Neisseria meningitidis The first comparative genomics study to S fimbriated Escherichia coli to isolated brain
in human brain microvascular endothelial cells. Mol. demonstrate that E. coli K1-derived genomic microvascular endothelial cells. Am. J. Pathol. 145,
Microbiol. 46, 933–946 (2002). islands contribute to meningitis. 1228–1236 (1994).
23. Jong, A. Y., Stins, M. F., Huang, S. H. & Kim, K. S. 43. Whitney, C. G. et al. Decline in invasive 62. Prasadarao, N. V., Wass, C. A. & Kim, K. S.
Traversal of Candida albicans across human pneumococcal disease after the introduction of Endothelial cell GlcNAcβ1–4 GlcNAc epitopes for
blood–brain barrier in vitro. Infect. Immun. 69, protein-polysaccharide conjugate vaccine. N. Engl. J. outer membrane protein A enhance traversal of
4536–4544 (2001). Med. 348, 1737–1746 (2003). Escherichia coli across the blood–brain barrier.
24. Chang, Y. C. et al. Cryptococcal yeast cells invade the 44. Tsai, C. J., Griffin, M. R., Pekka Nuorti, J. & Grijalva, Infect. Immun. 64, 154–160 (1996).
central nervous system via transcellular penetration C. G. Changing epidemiology of pneumococcal 63. Charland, N. et al. Streptococcus suis serotype 2
of the blood–brain barrier. Infect. Immun. 72, meningitis after the introduction of pneumococcal interactions with human brain microvascular
4985–4995 (2004). conjugate vaccine in the United States. Clin. Infect. endothelial cells. Infect. Immun. 68, 637–643 (2000).
The first study to show transcellular penetration Dis. 46, 1664–1672 (2008). 64. Hoffmann, I., Eugene, E., Nassif, X., Couraud, P. &
of the blood–brain barrier by C. neoformans. 45. Moore, M. R. et al. Population snapshot of emergent Bourdoulous, S. Activation of ErbB2 receptor
25. Masocha, W., Rottenberg, M. E. & Kristensson, K. Streptococcus pneumoniae serotype 19A in the tyrosine kinase supports invasion of endothelial cells
Migration of African trypanosomes across the United States, 2005. J. Infect. Dis. 197, by Neisseria meningitidis. J. Cell Biol. 155,
blood–brain barrier. Physiol. Behav. 92, 110–114 1016–1027 (2008). 133–145 (2001).
(2007). 46. Borrow, R. & Miller, E. Long-term protection in 65. Teng, C. H. et al. Escherichia coli K1 RS218 interacts
26. Grab, D. J. et al. African trypanosome interactions children with meningococcal C conjugate vaccination: with human brain microvascular endothelial cells via
with an in vitro model of the human blood–brain lessons learned. Expert Rev. Vaccines 5, 851–857 type 1 fimbria bacteria in the fimbriated state.
barrier. J. Parasitol. 90, 970–979 (2004). (2006). Infect. Immun. 73, 2923–2931 (2005).
27. Grab, D. et al. Borrelia burgdorferi, host-derived 47. Huang, S. H. et al. Escherichia coli invasion of brain Used DNA microarrays to identify type 1 fimbriae
proteases, and the blood–brain barrier. Infect. microvascular endothelial cells in vitro and in vivo: in E. coli that bind to HBMECs.
Immun. 73, 1014–1022 (2005). molecular cloning and characterization of invasion 66. Khan, N. A. et al. Outer membrane protein A and
28. Sethi, N., Sondey, M., Bai, Y., Kim, K. S. & Cadavid, D. gene ibe10. Infect. Immun. 63, 4470–4475 (1995). cytotoxic necrotizing factor‑1 use diverse signaling
Interaction of a neurotropic strain of Borrelia First identification of the E. coli gene that mechanisms for Escherichia coli K1 invasion of
turicatae with the cerebral microcirculation system. contributes to the invasion of HBMECs and human brain microvascular endothelial cells. Microb.
Infect. Immun. 74, 6408–6418 (2006). penetration into the brain. Pathog. 35, 35–42 (2003).
29. Drevets, D. A., Leenen P. J. & Greenfield, R. A. 48. Huang, S. H. et al. Identification and 67. Khan, N. A., Kim, Y., Shin, S. & Kim, K. S.
Invasion of the central nervous system by characterization of an Escherichia coli invasion gene FimH-mediated Escherichia coli K1 invasion of
intracellular bacteria. Clin. Microbiol. Rev. 17, locus, ibeB, required for penetration of brain human brain microvascular endothelial cells. Cell.
323–347 (2004). microvascular endothelial cells. Infect. Immun. 67, Microbiol. 9, 169–178 (2007).
30. Greiffenberg, L., Goebel, W., Kim, K. S. & Kuhn, M. 2103–2109 (1999). 68. Shin, S., Lu, G., Cai, M. & Kim, K. S. Escherichia coli
Interaction of Listeria monocytogenes with human 49. Khan, N. A. et al. Cytotoxic necrotizing factor-1 outer membrane protein A adheres to human brain
brain microvascular endothelial cells: InlB-dependent contributes to Escherichia coli K1 invasion of the microvascular endothelial cells. Biochem. Biophys.
invasion, long-term intracellular growth, and spread central nervous system. J. Biol. Chem. 277, Res. Commun. 330, 1199–1204 (2005).
from macrophages to endothelial cells. Infect. 15607–15612 (2002). 69. Prasadarao, N. V. et al. Cloning and expression of
Immun. 66, 5260–5267 (1998). 50. Wang, Y., Huang, S. H., Wass, C. & Kim, K. S. The the Escherichia coli K1 outer membrane protein A
31. Jain, S. K., Paul-Satyaseela, M., Lamichhane, G., gene locus yijP contributes to Escherichia coli K1 receptor, a gp96 homologue. Infect. Immun. 71,
Kim, K. S. & Bishai, W. R. Mycobacterium invasion of brain microvascular endothelial cells. 1680–1688 (2003).
tuberculosis invasion and traversal across an in vitro Infect. Immun. 67, 4751–4756 (1999). 70. Cabanes, D. et al. Gp96 is a receptor for a novel
human blood–brain barrier as a pathogenic 51. Wang, Y. & Kim, K. S. Role of OmpA and IbeB in Listeria monocytogenes virulence factor, Vip, a
mechanism for central nervous system tuberculosis. Escherichia coli invasion of brain microvascular surface protein. EMBO J. 24, 2827–2838 (2005).
J. Infect. Dis. 193, 1287–1295 (2006). endothelial cells in vitro and in vivo. Pediatr. Res. 71. Teng, C.-H. et al. Effects of ompA deletion on
The first paper to show transcellular penetration 51, 559–563 (2002). expression of type 1 fimbriae in Escherichia coli K1
of HBMECs by M. tuberculosis. 52. Prasadarao, N. V., Wass, C. A., Stins, M., Shimada, H. strain RS218 and on the association of E. coli with
32. Be, N. A. et al. Bacterial genetic determinants for & Kim, K. S. Outer membrane protein A-promoted human brain microvascular endothelial cells. Infect.
Mycobacterium tuberculosis invasion and survival in actin condensation of brain microvascular Immun. 74, 5609–5616 (2006).
the central nervous system. J. Infect. Dis. (in the endothelial cells is required for Escherichia coli 72. Badger, J., Wass, C., Weissman, S. & Kim, K. S.
press). invasion. Infect. Immun. 67, 5775–5783 (1999). Application of signature-tagged mutagenesis for
nature reviews | microbiology volume 6 | august 2008 | 633
© 2008 Macmillan Publishers Limited. All rights reserved.
REVIEWS
identification of Escherichia coli K1 genes that 91. Niemann, H. H. et al. Structure of the human Haemophilus influenzae type b capsule on blood
contribute to invasion of human brain microvascular receptor tyrosine kinase Met in complex with the brain barrier permeability during experimental
endothelial cells. Infect. Immun. 68, 5056–5061 Listeria invasion protein InIB. Cell 130, 235–246 meningitis in the rat. J. Clin. Invest. 82, 102–109
(2000). (2007). (1988).
73. Huang, S.-H., Wan, Z.-S., Chen, Y.-H., Jong, A. Y. & 92. Biswas, A. K. et al. Plasmodium falciparum uses 112. Vadeboncoeur, N., Segura, M., Al-Numani, D.,
Kim, K. S. Further characterization of Escherichia gC1qR/HABP1/p32 as a receptor to bind to vascular Vanier, G. & Gottschalk, M. Pro-inflammatory
coli brain microvascular endothelial cell invasion endothelium and for platelet-mediated clumping. cytokine and chemokine release by human brain
gene ibeA by deletion, complementation, and PloS Pathog. 3, 1271–1280 (2007). microvascular endothelial cells stimulated by
protein expression. J. Infect. Dis. 183, 1071–1078 93. Cundell, D. R., Gerard, N. P., Gerard, C., Idanpaan- Streptococcus suis serotype 2. FEMS Immunol. Med.
(2001). Heikkila, I. & Tuomanen, E. I. Streptococcus Microbiol. 35, 49–58 (2003).
74. Nemani, P. V., Wass, C. A., Huang, S.-H. & Kim, K. S. pneumoniae anchor to activated human cells by the 113. Frigerio, S. et al. Immunocompetence of human
Identification and characterization of a novel Ibe10 receptor for platelet-activating factor. Nature 377, microvascular brain endothelial cells: cytokine
binding protein that contributes to Escherichia coli 435–438 (1995). regulation of IL‑1β, MCP‑1, IL‑10, sICAM‑1 and
invasion of brain microvascular endothelial cells. 94. Radin, J. N. et al. β‑arrestin 1 participates in sVCAM‑1. J. Neurol. 245, 727–730 (1998).
Infect. Immun. 67, 1131–1138 (1999). platelet-activating factor receptor-mediated 114. Medana, I. M. & Turner, G. D. Human cerebral
75. Flatau, G. et al. Toxin-induced activation of the endocytosis of Streptococcus pneumoniae. Infect. malaria and the blood–brain barrier. Int. J.
G protein p21 Rho by deamidation of glutamine. Immun. 73, 7827–7835 (2005). Parasitol. 36, 555–568 (2006).
Nature 387, 729–733 (1997). 95. Weiser, J. N. et al. Phosphorylcholine on the 115. van der Heyde, H. C., Nolan, J., Combes, V.,
76. Schmidt, G. et al. Gln 63 of Rho is deamidated by lipopolysaccharide of Haemophilus influenzae Gramaglia, I. & Grau, G. E. A unified hypothesis for
Escherichia coli cytotoxic necrotizing factor‑I. Nature contributes to persistence in the respiratory tract the genesis of cerebral malaria: sequestration,
387, 725–729 (1997). and sensitivity to serum killing mediated by inflammation and hemostasis leading to
77. Contamin, S. et al. The p21 Rho-activating toxin C‑reactive protein. J. Exp. Med. 187, 631–640 microcirculatory dysfunction. Trends Parasitol. 22,
cytotoxic necrotizing factor 1 is endocytosed by a (1998). 503–508 (2006).
clathrin-independent mechanism and enters the cytosol 96. Cabellos, C. et al. Differing roles of platelet- 116. Tripathi, A. K., Sullivan, D. J. & Stins, M. F.
by an acidic-dependent membrane translocation step. activating factor during inflammation of the lung and Plasmodium falciparum-infected erythrocytes
Mol. Biol. Cell 11, 1775–1787 (2000). subarachnoid space. The special case of decrease the integrity of human blood–brain barrier
78. Chung, J. W. et al. 37-kDa laminin receptor Streptococcus pneumoniae. J. Clin. Invest. 90, endothelial cell monolayers. J. Infect. Dis. 195,
precursor modulates cytotoxic necrotizing factor 1 612–618 (1992). 942–950 (2007).
‑mediated RhoA activation and bacterial uptake. 97. Nassif, X., Pujol, C., Morand, P. & Eugene, E. 117. Kugler, S. et al. Pertussis toxin transiently affects
J. Biol. Chem. 278, 16857–16862 (2003). Interactions of pathogenic Neisseria with host cells. barrier integrity, organelle organization and
The first study to identify 37 LRP as the receptor Is it possible to assemble the puzzle? Mol. transmigration of monocytes in a human brain
for CNF1. Microbiol. 32, 1124–1132 (1999). microvascular endothelial cell barrier model. Cell.
79. Massia, S. P., Rao, S. S. & Hubbell, J. A. Covalently 98. Johansson, L. et al. CD46 in meningococcal disease. Microbiol. 9, 619–632 (2007).
immobilized laminin peptide Tyr‑Ile‑Gly‑Ser‑Arg Science 301, 373–375 (2003). 118. Perfect, J. R. & Casadevall, A. Cryptococcus. Infect.
(YIGSR) supports cell spreading and co-localization 99. Capecchi, B. et al. Neisseria meningitis NadA is a Dis. Clin. North Am. 4, 837–874 (2002).
of the 67-kilodalton laminin receptor with α-actinin new invasin which promotes bacterial adhesion to 119. Chretien, F. et al. Pathogenesis of cerebral
and vinculin. J. Biol. Chem. 268, 8053–8059 and penetration into human epithelial cells. Mol. Cryptococcus neoformans infection after fungemia.
(1993). Microbiol. 55, 687–698 (2005). J. Infect. Dis. 4, 522–530 (2002).
80. Kim, K. J., Chung, J. W. & Kim, K. S. 67-kDa 100. Plant, L. et al. Lipooligosaccharide structure 120. Olszewski, M. A. et al. Urease expression by
laminin receptor promotes internalization of contributes to multiple steps in the virulence of Cryptococcus neoformans promotes microvascular
cytotoxic necrotizing factor 1‑expressing Neisseria meningitidis. Infect. Immun. 74, sequestration, thereby enhancing central nervous
Escherichia coli K1 into human brain microvascular 1360–1367 (2006). system invasion. Am. J. Pathol. 164, 1761–1771
endothelial cells. J. Biol. Chem. 280, 1360–1368 101. Manchester, M. et al. Clinical isolates of measles (2004).
(2005). virus use CD46 as a cellular receptor. J. Virol. 74, 121. Noverr, M. C., Williamson, P. R., Fajardo, R. S. &
81. Ludwig, G. V., Kondig, J. P. & Smith, J. F. A putative 3967–3974 (2000). Huffnagle, G. B. CNLAC1 is required for
receptor for Venezuelan equine encephalitis virus 102. Gaggar, A., Shayakhmetov, D. M. & Lieber, A. extrapulmonary dissemination of Cryptococcus
from mosquito cells. J. Virol. 70, 5592–5599 CD46 is a cellular receptor for group B adenoviruses. neoformans but not pulmonary persistence. Infect.
(1996). Nature Med. 9, 1408–1412 (2003). Immun. 72, 1693–1699 (2004).
82. Gauczynski, S. et al. The 37-kDa/67-kDa laminin 103. Santoro, F. et al. Interaction of glycoprotein H of 122. Noveer, M. C., Cox, G. M., Perfect, J. R. &
receptor acts as the cell-surface receptor for the human herpesvirus 6 with the cellular receptor Huffnagle, G. B. Role of PLB1 in pulmonary
cellular prion protein. EMBO J. 20, 5863–5875 CD46. J. Biol. Chem. 278, 25964–25969 (2003). inflammation and cryptococcal eicosanoid
(2001). 104. Rich, A. R. & McCordock, H. A. The pathogenesis of production. Infect. Immun. 71, 1538–1547
83. Thepparit, C. & Smith, D. R. Serotype-specific entry tuberculous meningitis. Bull. Johns Hopkins Hosp. (2003).
of dengue virus into liver cells: identification of the 52, 5–37 (1933). 123. Santangelo, R. et al. Role of extracellular
37-kilodalton/67-kilodalton high-affinity laminin 105. Thomas, D. D. et al. Treponema pallidum invades phospholipases and mononuclear phagocytes in
receptor as a dengue virus serotype 1 receptor. intercellular junctions of endothelial cell dissemination of cryptococcosis in a murine model.
J. Virol. 78, 12647–12656 (2004). monolayers. Proc. Natl Acad. Sci. USA 85, Infect. Immun. 72, 2229–2239 (2004).
84. Akache, B. et al. The 37/67-kilodalton laminin receptor 3608–3612 (1988). 124. Shea, J. M., Kechichian, T. B., Luberto, C. & Del
is a receptor for adeno-associated virus serotypes 8, 2, 106. Zhang, G. W., Khan, N. A., Kim, K. J., Stins, M. & Poeta, M. The cryptococcal enzyme inositol
3, and 9. J. Virol. 80, 9831–9836 (2006). Kim, K. S. Transforming growth factor‑β increases phosphosphingolipid-phospholipase C confers
85. Tenenbaum, T. et al. Streptococcus agalactiae Escherichia coli K1 adherence, invasion and resistance to the antifungal effects of macrophages
invasion of human brain microvascular endothelial transcytosis in human brain microvascular and promotes fungal dissemination to the central
cells is promoted by the laminin-binding protein endothelial cells. Cell Tissue Res. 309, 281–286 nervous system. Infect. Immun. 74, 5977–5988
Lmb. Microbes Infect. 9, 714–720 (2007). (2002). (2006).
86. Tenenbaum, T., Bloier, C., Adam, R., Reinscheid, 107. Galanakis, E., Di Cello, F., Paul-Satyaseela, M. &
D. J. & Schroten, H. Adherence to and invasion of Kim, K. S. Escherichia coli K1 induces IL‑8 Acknowledgements
human brain microvascular endothelial cells are expression in human brain microvascular The information contained in this Review is derived from stud-
promoted by fibrinogen-binding protein FbsA of endothelial cells. Eur. Cytokine Netw. 17, 260–265 ies carried out by members of the author’s laboratory and his
Streptococcus agalactiae. Infect. Immun. 73, (2006). collaborators’ laboratories. Work in the laboratory of K.S.K.
4404–4409 (2005). 108. Sokolova, O. et al. Interaction of Neisseria was supported by National Institutes of Health grants.
87. Doran, K. S. et al. Blood–brain barrier invasion by meningitidis with human brain microvascular
group B Streptococcus depends upon proper cell- endothelial cells: role of MAP- and tyrosine kinases
surface anchoring of lipoteichoic acid. J. Clin. Invest. in invasion and inflammatory cytokine release. Cell. DATABASES
115, 2499–2507 (2005). Microbiol. 6, 1153–1166 (2004). Entrez Genome Project: http://www.ncbi.nlm.nih.gov/
88. Maisey, H. C., Hensler, M., Nizet, V. & Doran, K. S. 109. Tuomanen, E., Saukkonen, K., Sande, S., Cioffe, C. & entrez/query.fcgi?db=genomeprj
Group B streptococcal pilus proteins contribute to Wright, S. D. Reduction of inflammation, tissue Borrelia burgdorferi | Borrelia turicatae | Candida albicans |
adherence to and invasion of brain microvascular damage and mortality in bacterial meningitis in Cryptococcus neoformans | Escherichia coli | Haemophilus
endothelial cells. J. Bacteriol. 189, 1464–1467 rabbits treated with monoclonal antibodies against influenzae | Listeria monocytogenes | Mycobacterium
(2007). adhesion-promoting receptors of leukocytes. J. Exp. smegmatis | Mycobacterium tuberculosis | Neisseria
89. Braun, L., Ghebrehiwet, B. & Cossart P. gC1q‑R/p32, Med. 170, 959–968 (1989). meningitidis | Plasmodium falciparum | Streptococcus
a C1q-binding protein, is a receptor for the InlB 110. Tauber, M. G., Borschberg, U. & Sande, M. A. agalactiae | Streptococcus pneumoniae | Streptococcus suis |
invasion protein of Listeria monocytogenes. EMBO J. Influence of granulocytes on brain edema, Toxoplasma gondii | Treponema pallidum
19, 1458–1466 (2000). intracranial pressure, and cerebrospinal fluid Entrez Protein: http://www.ncbi.nlm.nih.gov/entrez/query.
90. Shen, Y., Naujokas, M., Park, M. & Ireton, K. concentrations of lactate and protein in experimental fcgi?db=protein
InIB-dependent internalization of Listeria is mediated meningitis. J. Infect. Dis. 157, 456–464 (1988). CNF1 | FbsA | IbeA | IbeB | IL‑8 | InlB | Lmb | NadA | OmpA
by the Met receptor tyrosine kinase. Cell 103, 111. Lesse, A. J., Moxon, E. R., Zwahten, A. & Scheid, All links are active in the online pdf
501–510 (2000). W. M. Role of cerebrospinal fluid pleocytosis and
634 | august 2008 | volume 6 www.nature.com/reviews/micro
© 2008 Macmillan Publishers Limited. All rights reserved.
You might also like
- Neurosciences Research: Volume 3From EverandNeurosciences Research: Volume 3S EhrenpreisNo ratings yet
- Kim 2008Document25 pagesKim 2008jose mendozaNo ratings yet
- Pathogenesis of Bacterial Meningitis: From Bacteraemia To Neuronal InjuryDocument10 pagesPathogenesis of Bacterial Meningitis: From Bacteraemia To Neuronal InjurymjeanjNo ratings yet
- How Do Extracellular Pathogens Cross The Blood-Brain Barrier?Document6 pagesHow Do Extracellular Pathogens Cross The Blood-Brain Barrier?Jade LoberianoNo ratings yet
- Cshperspectmed BAC A012393Document14 pagesCshperspectmed BAC A012393RaffaharianggaraNo ratings yet
- Mechanism of Meningeal Invasion by Neisseria MeningitidisDocument10 pagesMechanism of Meningeal Invasion by Neisseria MeningitidisRaffaharianggaraNo ratings yet
- To The Brain and Back Migratory Paths of DendriticDocument15 pagesTo The Brain and Back Migratory Paths of DendriticarriliaNo ratings yet
- CNS - Infections F2022 OVER VIEWDocument18 pagesCNS - Infections F2022 OVER VIEWMadison MillwoodNo ratings yet
- Pathophysiology of Meningococcal Meningitis and SepticaemiaDocument8 pagesPathophysiology of Meningococcal Meningitis and SepticaemiaEugen TarnovschiNo ratings yet
- Cerebral OedemDocument7 pagesCerebral OedemSagita NindraNo ratings yet
- New 1Document12 pagesNew 1Muhammad SyaifullahNo ratings yet
- FEMSminireviewDocument11 pagesFEMSminireviewRaffaharianggaraNo ratings yet
- Immunopathogenesis of Brain Abscess Journal of Neuroinflammation 2004, 116Document10 pagesImmunopathogenesis of Brain Abscess Journal of Neuroinflammation 2004, 116VI VqdNo ratings yet
- Host-Pathogen Interactions in Bacterial MeningitisDocument25 pagesHost-Pathogen Interactions in Bacterial MeningitisEugen TarnovschiNo ratings yet
- Centralnervoussystem Lesionsin Immunocompromisedpatients: Robert Y. Shih,, Kelly K. KoellerDocument15 pagesCentralnervoussystem Lesionsin Immunocompromisedpatients: Robert Y. Shih,, Kelly K. Koelleralejandro echeverriNo ratings yet
- Centralnervoussystem Lesionsin Immunocompromisedpatients: Robert Y. Shih,, Kelly K. KoellerDocument15 pagesCentralnervoussystem Lesionsin Immunocompromisedpatients: Robert Y. Shih,, Kelly K. Koelleralejandro echeverriNo ratings yet
- Brain Abscess: State-Of-The-Art Clinical ArticleDocument17 pagesBrain Abscess: State-Of-The-Art Clinical ArticleAwais KhanNo ratings yet
- Brain Tuberculomas, Tubercular Meningitis, and Post-Tubercular Hydrocephalus in ChildrenDocument5 pagesBrain Tuberculomas, Tubercular Meningitis, and Post-Tubercular Hydrocephalus in ChildrenDave VentraNo ratings yet
- Research Open Access: Charlotte Schmitt, Nathalie Strazielle and Jean-François Ghersi-EgeaDocument15 pagesResearch Open Access: Charlotte Schmitt, Nathalie Strazielle and Jean-François Ghersi-Egeamihail.boldeanuNo ratings yet
- Brain Immunology and Immunotherapy in Brain TumoursDocument14 pagesBrain Immunology and Immunotherapy in Brain TumoursAnonymous QLadTClydkNo ratings yet
- Could Proteomic Research Deliverthe Next GenerationofDocument11 pagesCould Proteomic Research Deliverthe Next GenerationofHafriliantika RamadhaniNo ratings yet
- Meningitis: Review ArticleDocument19 pagesMeningitis: Review ArticleYo Me100% (1)
- Brain Abscess: State-Of-The-Art Clinical ArticleDocument17 pagesBrain Abscess: State-Of-The-Art Clinical ArticleIqbal AbdillahNo ratings yet
- The Pathogenesis of Tuberculous MeningitisDocument14 pagesThe Pathogenesis of Tuberculous MeningitisGladys SuwantiNo ratings yet
- Impact of Aging On Meningeal Gene Expression: Research Open AccessDocument14 pagesImpact of Aging On Meningeal Gene Expression: Research Open AccessCamila Alejandra Inostroza RiveraNo ratings yet
- CNS Infiltration of Peripheral Immune Cells - D-Day For Neurodegenerative DiseaseDocument14 pagesCNS Infiltration of Peripheral Immune Cells - D-Day For Neurodegenerative DiseaseFrancesca GrassivaroNo ratings yet
- Pathogenesis of The Central Nervous System InfectionDocument44 pagesPathogenesis of The Central Nervous System InfectionMira ApriliaNo ratings yet
- Pathogenesis Infection of CnsDocument4 pagesPathogenesis Infection of CnshadiNo ratings yet
- J Neurol Neurosurg Psychiatry-2000-Newton-433-41Document10 pagesJ Neurol Neurosurg Psychiatry-2000-Newton-433-41Joy SanjayaNo ratings yet
- Management of Abcess CerebriDocument6 pagesManagement of Abcess Cerebricamelia musaadNo ratings yet
- Recent Insights Into CCMDocument1 pageRecent Insights Into CCMSavannah HollisNo ratings yet
- Cerebral Malaria Immunopathology PDFDocument11 pagesCerebral Malaria Immunopathology PDFAurelia WetondieNo ratings yet
- Temporal and Spatial Dynamics of Cerebral Immune Cell Accumulation in StrokeDocument9 pagesTemporal and Spatial Dynamics of Cerebral Immune Cell Accumulation in StrokeLeon PalomeraNo ratings yet
- Immunotherapy For Brain Tumors: Ournal of Linical NcologyDocument9 pagesImmunotherapy For Brain Tumors: Ournal of Linical NcologyAsti YumnaNo ratings yet
- Objetivo 3 Móvil PDFDocument26 pagesObjetivo 3 Móvil PDFnawal akarmaniNo ratings yet
- Description of The Disease MeningitisDocument3 pagesDescription of The Disease MeningitisLance SilvaNo ratings yet
- Paper 6Document6 pagesPaper 6William AndaroNo ratings yet
- Living Proof of Activity of Extracellular VesiclesDocument17 pagesLiving Proof of Activity of Extracellular VesiclesRamona AnaNo ratings yet
- Neuroimmune Communication: EditorialDocument1 pageNeuroimmune Communication: EditorialCassie MinorNo ratings yet
- Cerebral MalariaDocument16 pagesCerebral MalariaNLailyrahmaNo ratings yet
- Knjiga Za WNVDocument19 pagesKnjiga Za WNVellvira1991No ratings yet
- Neuroscience - The Brain and Spiral CordDocument4 pagesNeuroscience - The Brain and Spiral CordAnn MutisyaNo ratings yet
- The Blood-Brain BarrierDocument13 pagesThe Blood-Brain BarrierCabinet VeterinarNo ratings yet
- Bacterial MeningitisDocument26 pagesBacterial MeningitisRai HannaNo ratings yet
- Fnmol 12 00057Document9 pagesFnmol 12 00057Uziko 10No ratings yet
- NeuroiflammationDocument9 pagesNeuroiflammationGabrielaNo ratings yet
- Wright 2008Document17 pagesWright 2008Ardi PratamaNo ratings yet
- DC 1 PDFDocument25 pagesDC 1 PDFRebecca SandoicaNo ratings yet
- Pathogens 11 01212Document13 pagesPathogens 11 01212Bapa akuNo ratings yet
- Macropinocytosis and SARS-CoV-2 Cell EntryDocument12 pagesMacropinocytosis and SARS-CoV-2 Cell EntryKarla SegovianoNo ratings yet
- Central Nervous System Infection in The PediatricDocument5 pagesCentral Nervous System Infection in The PediatricJeriz Marie GamboaNo ratings yet
- Mold Infections of The Central Nervous SystemDocument15 pagesMold Infections of The Central Nervous SystemJohn TusselNo ratings yet
- Atb Meningitis Coid 2018Document12 pagesAtb Meningitis Coid 2018Danny JacobusNo ratings yet
- The Neurological ManifestationsDocument13 pagesThe Neurological ManifestationsandreaNo ratings yet
- 2.lab Systemic Path CNS Exercise NeucomDocument6 pages2.lab Systemic Path CNS Exercise NeucomElysa Dela CruzNo ratings yet
- Extraparenchymal (Racemose) Neurocysticercosis and Its Multitude Manifestations: A Comprehensive ReviewDocument9 pagesExtraparenchymal (Racemose) Neurocysticercosis and Its Multitude Manifestations: A Comprehensive ReviewalameluNo ratings yet
- Infectious Diseases Pharmacotherapy: Lesson 5 Central Nervous System InfectionDocument63 pagesInfectious Diseases Pharmacotherapy: Lesson 5 Central Nervous System Infectionbest batiNo ratings yet
- Net InductionDocument18 pagesNet InductionManovriti ThakurNo ratings yet
- 2019 # Ribeiro Regulation of Innate Immune Responses by PlateletsDocument9 pages2019 # Ribeiro Regulation of Innate Immune Responses by PlateletsMaria Eduarda AquinoNo ratings yet
- Infectious Biology 5Document2 pagesInfectious Biology 5JohnchubyNo ratings yet
- Algal-Bacterial Consortia For Bioproduct Generation andDocument15 pagesAlgal-Bacterial Consortia For Bioproduct Generation andJEAN CERNA SOLISNo ratings yet
- The Role of Microbial Motility andDocument11 pagesThe Role of Microbial Motility andJEAN CERNA SOLISNo ratings yet
- China's New National Standard For Drinking Water TDocument1 pageChina's New National Standard For Drinking Water TJEAN CERNA SOLISNo ratings yet
- Microbial Evolution and TransitionsDocument16 pagesMicrobial Evolution and TransitionsJEAN CERNA SOLISNo ratings yet
- China's One Child Policy Itself Leads To Forced AbDocument1 pageChina's One Child Policy Itself Leads To Forced AbJEAN CERNA SOLISNo ratings yet
- Address Physical Inactivity, But Avoid Stressing H - 17Document1 pageAddress Physical Inactivity, But Avoid Stressing H - 17JEAN CERNA SOLISNo ratings yet
- America's-Future LancetDocument1 pageAmerica's-Future LancetJEAN CERNA SOLISNo ratings yet
- Helicobacter Pylori: An Up To Date Overview On The VirulenceDocument18 pagesHelicobacter Pylori: An Up To Date Overview On The VirulenceJEAN CERNA SOLISNo ratings yet
- Helicobacter Pylori Infection in FunctionalDocument7 pagesHelicobacter Pylori Infection in FunctionalJEAN CERNA SOLISNo ratings yet
- InfluenzaDocument24 pagesInfluenzanurul fatma diyanaNo ratings yet
- Bodies in PathologyDocument2 pagesBodies in PathologySreekanth KrishnamurthyNo ratings yet
- HapDocument15 pagesHapWinariieeyy Nayy100% (1)
- Techtalk August2010Document2 pagesTechtalk August2010Abu KhalidNo ratings yet
- BCSC1718 S09 PDFDocument15 pagesBCSC1718 S09 PDFrisaNo ratings yet
- STD 101Document42 pagesSTD 101Clarice BedicoNo ratings yet
- PathologyDocument48 pagesPathologyAjay DivvelaNo ratings yet
- UNICEF - 2010 - HandBook For Cold Chain ManagersDocument112 pagesUNICEF - 2010 - HandBook For Cold Chain ManagersasmintehminaNo ratings yet
- Q22019 CD Bulletin Vol 10 Issue 2 22 Dec 2019Document28 pagesQ22019 CD Bulletin Vol 10 Issue 2 22 Dec 2019Leo TrileoNo ratings yet
- Guia Ingles - Grado 11Document8 pagesGuia Ingles - Grado 11Juan CuervoNo ratings yet
- EPI ManualDocument73 pagesEPI ManualRich Arocena100% (1)
- Conjunctivitis 1Document28 pagesConjunctivitis 1Nrs Sani Sule MashiNo ratings yet
- June 8, 2018 Strathmore TimesDocument24 pagesJune 8, 2018 Strathmore TimesStrathmore TimesNo ratings yet
- Approach To A Patient With ParaplegiaDocument31 pagesApproach To A Patient With ParaplegiaFaisal Qureshi75% (4)
- Food Code HaccpDocument37 pagesFood Code HaccpjucabrerasoNo ratings yet
- Aseptic Meningitis: Exams and TestsDocument8 pagesAseptic Meningitis: Exams and TestsJoylyn Sagon VergaraNo ratings yet
- Antibody Identification: Part 1: The Basics A Blood Bank Guy Video PodcastDocument18 pagesAntibody Identification: Part 1: The Basics A Blood Bank Guy Video PodcastNguyen Thi Phuong NhiNo ratings yet
- Portfolio Clinical Case Study 3 Lymphoma FinalDocument27 pagesPortfolio Clinical Case Study 3 Lymphoma Finalapi-277136509No ratings yet
- Chemotherapeutic AgentsDocument53 pagesChemotherapeutic AgentsGrape JuiceNo ratings yet
- Executive Briefer EreidDocument2 pagesExecutive Briefer Ereidjohnllenalcantara50% (2)
- Usha Bansal - 112010120007279Document3 pagesUsha Bansal - 112010120007279ramanlalbansalNo ratings yet
- Disease Project Handout and RubricDocument1 pageDisease Project Handout and Rubricapi-409240655No ratings yet
- Swine Flu VaccineDocument4 pagesSwine Flu VaccineShailaja DesaiNo ratings yet
- Switch Antibiotic IV To OralDocument2 pagesSwitch Antibiotic IV To OralRidwan Ibnu BahariNo ratings yet
- Log (Protonated Form) (Non-Protonated Form) : Pka - PHDocument5 pagesLog (Protonated Form) (Non-Protonated Form) : Pka - PHLinh HoangNo ratings yet
- Imunisasi Record - KennyDocument1 pageImunisasi Record - KennyrifiNo ratings yet
- Pisa Science QuestionsDocument8 pagesPisa Science QuestionsYuvi Ghaly100% (1)
- Hes 032 Lec Long Quiz 1 BSN 2 A11Document24 pagesHes 032 Lec Long Quiz 1 BSN 2 A11Sabrina MascardoNo ratings yet
- Clinical Pharmacy of AntibioticsDocument53 pagesClinical Pharmacy of AntibioticsEkanita DesianiNo ratings yet
- Differential Leukocytes CountDocument2 pagesDifferential Leukocytes CountSalmanNo ratings yet
- Love Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)From EverandLove Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)Rating: 3 out of 5 stars3/5 (1)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedFrom EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedRating: 4.5 out of 5 stars4.5/5 (82)
- The Age of Magical Overthinking: Notes on Modern IrrationalityFrom EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityRating: 4 out of 5 stars4/5 (32)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionFrom EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionRating: 4 out of 5 stars4/5 (404)
- ADHD is Awesome: A Guide to (Mostly) Thriving with ADHDFrom EverandADHD is Awesome: A Guide to (Mostly) Thriving with ADHDRating: 5 out of 5 stars5/5 (3)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsFrom EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsNo ratings yet
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsFrom EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsRating: 5 out of 5 stars5/5 (1)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsFrom EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsRating: 4 out of 5 stars4/5 (4)
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisFrom EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisRating: 3.5 out of 5 stars3.5/5 (2)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeFrom EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeRating: 2 out of 5 stars2/5 (1)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisFrom EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisRating: 4.5 out of 5 stars4.5/5 (42)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.From EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Rating: 4.5 out of 5 stars4.5/5 (110)
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeFrom EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeRating: 4.5 out of 5 stars4.5/5 (254)
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsFrom EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsRating: 4.5 out of 5 stars4.5/5 (170)
- Manipulation: The Ultimate Guide To Influence People with Persuasion, Mind Control and NLP With Highly Effective Manipulation TechniquesFrom EverandManipulation: The Ultimate Guide To Influence People with Persuasion, Mind Control and NLP With Highly Effective Manipulation TechniquesRating: 4.5 out of 5 stars4.5/5 (1412)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaFrom EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaRating: 4.5 out of 5 stars4.5/5 (266)
- To Explain the World: The Discovery of Modern ScienceFrom EverandTo Explain the World: The Discovery of Modern ScienceRating: 3.5 out of 5 stars3.5/5 (51)
- The Obesity Code: Unlocking the Secrets of Weight LossFrom EverandThe Obesity Code: Unlocking the Secrets of Weight LossRating: 4 out of 5 stars4/5 (6)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryFrom EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryRating: 4 out of 5 stars4/5 (46)
- The Marshmallow Test: Mastering Self-ControlFrom EverandThe Marshmallow Test: Mastering Self-ControlRating: 4.5 out of 5 stars4.5/5 (60)
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessFrom EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessRating: 4.5 out of 5 stars4.5/5 (328)
- Why We Die: The New Science of Aging and the Quest for ImmortalityFrom EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityRating: 4.5 out of 5 stars4.5/5 (6)
- How to ADHD: The Ultimate Guide and Strategies for Productivity and Well-BeingFrom EverandHow to ADHD: The Ultimate Guide and Strategies for Productivity and Well-BeingRating: 1 out of 5 stars1/5 (1)