Professional Documents
Culture Documents
CLS MED 23 24 XI Che Package 1 Level 2 Chapter 2
CLS MED 23 24 XI Che Package 1 Level 2 Chapter 2
Uploaded by
DarkAngel0 ratings0% found this document useful (0 votes)
26 views14 pagesCopyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
26 views14 pagesCLS MED 23 24 XI Che Package 1 Level 2 Chapter 2
CLS MED 23 24 XI Che Package 1 Level 2 Chapter 2
Uploaded by
DarkAngelCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 14
NZ) ea |
Chapter 2
Structure of Atom
SECTION -A
: :
2
vo Intensity of light
a cis he
Threshold frequency
IKE of electron increases after crossing threshold frequency]
2. Answer (2)
All the wavelength are in visible region ie., between 400 nm to 760 nm. Therefore, maximum wavelength line
will be 656 nm,
3. Answer (1)
KE
Ero
Total energy =-3.4 eV (Gen)
2 KE = 3.4 eV) = 43.4 eV
4. Answer (4)
As 1* excited state means n, = 2
For 5" excited state means n,
Electrons wil transit between 6" level to 2° level
No transition will be upto 1st level. Because no line will appear in Lyman series /., UV region.
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005. Phone : 011-47623456
66 Structure of Atom Solutions of Assignment (Levelt)
5. Answer (1)
Tline in the Balmer series means n, = 2,n,= 3
6. Answer (2)
(Egous =xeV (given)
Ena In=3 ecouse (2 excted stat)
be] ee
For He®, Z =
= 1096784 om
4
oae7axd ~ aae77g =22x10 om
[22x10 m|
8. Answer (2)
Third line means third excited state
ion =2 Balmer series (visible region)
n= Third line
Third line will appear when electron comes from 5! energy level to 2% level
9. Answer (1)
In H atom
g, = 21312 = 21312 g, = 21912 g, = 21312 e, = 2322
12 4 9 25 36
(E, — E,) > (E, — E,) > (Ey — E,)
[Alternatively as the distance from the nucleus increases the value of AE (energy difference between two shell)
decreases]
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005, Phone : 011-47623456
Solutions of Assignment (Level) Structure of Atom 67
10. Answer (3)
Largest amount of energy is required for the transition between 1 > =»
1 1
AE=he x7 he] | Lage the efarence betwee n, ann age wil be the valu of AE}
11, Answer (2)
circumference _ 2nr_n® 16
Sroumference _ Zit _ 2 <1.5x10" seconds
velocity v,
Time period =
Time period sn?
12, Answer (1)
First time of Paschen series n, = 3, n, = 4
a
ALR ye
a, 144 IR
Second line of Paschen series n, = 3, ny = 5
1_pft_4
tert
ie 5 25!
16k 225
225 2 TER
[> = 144 2304 _ 256]
ie 7R 1675 175
2.
as ny =n, are constants
more the nuclear charge smaller will be the A.
L?* have shorter wavelength
14, Answer (1)
AP = 10° kgms~!
axxap = 2
an
86x10 D5 210% m
1074x314
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005. Phone : 011-47623456
68 Structure of Atom Solutions of Assignment (Levelt)
15. Answer 2)
bse ay (Gwen)
a TF]
ig DP
‘hp = 2hq x 10-%m
hg 22x85 x 105
= 10% 10m 4 m= 100 om
= 107 m= 10 om
16. Answer (@)
In 3 means 4f|
for | = 3, m = -3, -2, -1, 0, 1, 2, 3 = 7 orbital
“Therefore, maximum 14 electons ae preset
17, Answer (1)
18. Answer (2)
Energy of an electron depends upon (n + /) value.
More the (n + value more wil bee energy
no m s (or)
ms 2 1 np 5
@4 2 4 np 6 Max (n +1). max. energy
(3) 4 1 0 12 5
ws 0 0 “1p 5
19. Answer (3)
Total number of electrons in X°
[Number of electrons in X= 22+ 3= 25)
Atomic number = 25
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005, Phone : 011-47623456
Solutions of Assignment (Level) Structure of Atom 69)
20. Answer (3)
ons having all the electron paired will be non-paramagnetic or diamagnetic
Ne*? 8 18%, 282, 2p* aii [4 2 unpaired electrons
2p
Be® 3 18%, 2s! 4 1 unpaired electron
25
Cr = 18 = 182, 25%, 2p8, 382, 3p? ss 0 unpaired electron
3p
‘As® 33 18%, 28, 2p°, 38%, 3p®, 4s%, 30, 4p? 411i 3 unpaired electrons
21. Answer (1)
Isoelectronic species have same number of electrons
(co=14e" NO®=14e"]
CN = 146° Ci =14 6° | [Al have same numberof electrons)
22. Answer (2)
(9. (9), and (9) are not posse
(i) n=2 I not equal to n not possible
@n=t s= 2 Not possible because m = ~1 where | = 0
w n=3 s= +12 Not possible because m = 3 is not for | = 2
23, Answer (3)
‘Any ofbital have maximum of two electrons with opposite spin
24. Answer (1)
For | = 3, m= -3, -2, -1, 0, +1, +2, +3 ie., 7 ombitals are present
25. Answer (3)
K = 19 = 18%, 282, 2p%, 35%, 3p%, 4s! last e~
Last electron 4st
ened m=0
26. Answer (1)
M shell means 3" orbit
Mn ='25 = 18’, 25°, 2p', 9s’, 3p", 4s’, 3c total 13 e in 3 orbit
8 5
Co = 27 = 1s%, 25%, 2p%, 352, 3p8, 482, 347 total 15 e in 3 orbit
Ni = 28 = 15?, 28%, 2p®, 35%, 3p%, 452, 3d® total 16 e in 3 orbit
Fe = 26 = 1s%, 2s?, 2p8, 35%, 3p%, 482, 3d® total 14 e in 3 orbit
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005. Phone : 011-47623456
70 Structure of Atom Solutions of Assignment (Levelt)
27. Answer (2)
= 3 [Not possible because value of | can never be equals to n]
28, Answer (2)
h
Orbital angular momentum = i(I+4)
For 4s electron, the value of |= 0 *, [orbital angular momentum = zero]
29. Answer (2)
Radial nodes = (n - 1-1)
for 3s, (3-0-1)=2; For 3p, (3-
30. Answer (3)
Energy = (n +1)
A=n=4 121 244125
B=n=4 |=0 =4+0=4
C=n=3) 1-2 =342=5
D=n=3) I=1 9 =341=4
‘According to Pauli exclusion principle
(1) Larger the (n + ); larger will be eneray
(2) Same value of (n +1) ; smaller n ; more will be eneray
31. Answer (4)
32. Answer (2)
Value of m = 0 for 3s, 2p, and 3d,»
33. Answer (3)
Total number of electron in subshell = 2(21 + 1), | = angular quantum number
2241) _
Number of electrons having same spin =
(a+)
2
[Because half e~ have clockwise and half ~ have anti clockwise spin]
34. Answer (4)
For 2nd period electronic configuration = 2%, 2p
If each orbital have 3e~ then electronic configuration = 2s°, 2p,°, 2p,2, 2p,
Total 12 e° will present
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005, Phone : 011-47623456
Solutions of Assignment (Level) Structure of Atom 71
SECTION - B
4. Answer (2)
+ Inan atom, all the five 3d orbitals are equal in energy in free state i.e., degenerate,
+ The shape of d,z_2 Is different then shape of dz
dey a,
y x
y x
+ The size of orbital depends on principal quantum number ‘n’ therefore all the five 3d orbitals are different
in size when compared to the respective 4d orbitals,
+ Shape of orbitals depends on azimuthal quantum number ‘therefore shapes of 4d orbitals are similar to
the respective 3d orbitals,
2. Answer (3)
fy
hae
Z
(Li)
“a(He")
LP") _@F 2
1058 3 “(@2F
3
= 105.85
(LP*) = 158.7 pm
3. Answer (1)
1 |_| | Subshell notation
2/0 25
2f4 2p
3 [0 35
3 [4 3p
3 [2 ad
4, Answer (1)
he
Energy of one photon = “= (a= 300m)
he
For one mole photons, = 5>xNy
6.626107 x3x10° x6,023x 102%
300x107
E = 3.99 x 10° J mol"
E
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005. Phone : 011-47623456
72 Structure of Atom
Kinetic energy = 1.68 x 10° J mot~?
W, = E- KE.
3,99 108 — 1.68 x 10°
= 2.31 « 108 J mor*
5. Answer (2)
Energy of electromagnetic radiation (E)
_ 3x10
= 7368x10°
6, Answer (4)
a =219.3m
MEL
Number of Protons = 71 = No. of Electrons
jass no. ~ No. of Protons
= 175-71 = 104
Number of Neutrons
7. Answer (2)
© Number of radial nodes =n —
Number of angular nodes
For 3s orbital,
= Number of radial nodes = 3 - 0
© Number of angular nodes = 0
8 Answer (4)
Total number of nodes = (n - 1)
3=n-1
ne4
Number of angular nodes = /= 3 > Fsubshell
Correct answer is 4f,
8, Answer (3)
= ay?
1, = 52.9 * (2)? pm
Solutions of Assignment (Levelt)
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005, Phone : 011-47623456
Solutions of Assignment (Level) Structure of Atom 73,
10. Answer (1)
(n+l) values for, 4d=4+2=6
Sp=54+1=6
f= 5+3=8
6p=6+1=7
Correct order of energy would be
5f> 6p > Sp > 4d
11, Answer (2)
In H-spectrum, Balmer series transitions fall in visible region.
12. Answer (4)
According to Hund's Rule of maximum multiplicity, the correct electronic configuration of N-atom is
ty (MILT t | t
is’ 2s
2p
n) MGW
Option (4) violates Hunct's Rule.
13. Answer (4)
Energy of 2s-orbital and 2p-orbital in case of hydrogen ike atoms is equal
14, Answer (1)
‘An orbital can accommodate maximum of 2 electrons with anti-parallel spins.
15. Answer (3)
z y
d, dap
16. Answer (1)
Fact.
17. Answer (2)
‘Angular momentum = irae = TRS
18, Answer (1)
Itrepresents 3p orbital
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005. Phone : 011-47623456
74° Structure of Atom Solutions of Assignment (Levelt)
19. Answer (4)
APE 8691 xO AOE 868 sgt yaa gory
x 48x10" 15
20. Answer (2)
21. Answer (3)
Value of m
22. Answer (2)
E
4 represents one orbital. Therefore, maximum number of electrons will be two,
3.0%10"7 nms-
6x10" §
h=S|
v
4.10? nm
2
= 0.5% 10? nm = 50 nm
23. Answer (3)
In (n= 1) 1% shell eis tightly held compared to
24, Answer (1)
Rb = 37 = [Ar] 4s", 30, 4p* last 5s° e-
(6" shell)
n=65,1=0,m=0,s=
a
2
25. Answer (3)
n=4
represents 4f subshell having 7 orbitals
‘Total number of electrons = 14
26, Answer (1)
Angular momentum = (1-4)
27. Answer (3)
Number of orbitals = n*] n = number of orbit
=4=16
28. Answer (3)
Te] he
y= Re) a, = BE -
iE aE E,=50eV
he
ay = he
5 a
fe 2
50 @)
he
B
fe
50
f= Oy
29. Answer (2)
Putting the value of n and calculating the (n + |) value
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005, Phone : 011-47623456
Solutions of Assignment (Level) Structure of Atom 75)
6s < 4 < Sd < 6p
(6+0) (443) (5+2) (6+1)
6 7 7 7
(lower energy) > (high energy)
30. Answer (1)
Because (E, ~E,) > (E, -E,) > (E,-E,) > (E,-E,)> (E,-£,)
As the difference is of one energy levels,
(E, -E,) have less energy
(Alternatively value of AE [difference between two successive energy level decreases] as the distance from the
ly ay
nucleus increases.)
31. Answer (3)
Lh _ 8.6x10kgm?s?-s
mv 0.66 kgx100 mis
32. Answer (4)
KE = Energy observed by molecule ~ Energy required to break one bond
0x10" my
= 44x1079J-4.0 x 107%
2
KE
0.4x10-* \-19,
Ke pratm =| 24250) foaxtos]
33. Answer (4)
34, Answer (2)
n=3
Value of m (orbital) depends upon fi.e., it cannot be more than I’, Therefore is wrong.
35. Answer (3)
36, Answer (2)
h
Axx Ap = 3
37. Answer (2)
38. Answer (4)
39. Answer (1)
laxscmav = | ay =—_h__
an| ’ Demxaxn
. 66x10 J-s .
OAT <9. 10 kg 4xa.14 = 5.788X10" mis
40. Answer (3)
41, Answer (4)
Av
372|
a | kd mol for hydrogen
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005. Phone : 011-47623456
76
Structure of Atom
42, Answer (3)
44,
2
ho 66x10" ia
SAmaV 4309,14x9, 1410 x APOE xt08
43. Answer)
p= BSS 0.1764
naver
Energy of electron when n= 1, y= 32 ‘aim
Evo of aletonwhonn = 96, = 1
45,
46.
47.
=2.0x10° 6631089148], a
‘Answer (4)
Solutions of Assignment (Levelt)
+1166 kJ/mol = 1166 x 10° J/mol = 1166 x 10" erg/mol
1936 * 10- erglatom
Gd have exceptional configuration e* will enter in 5d because 4fhave 7 electrons and have haf filed stability
[oa = Xe)™ 477 50°65"
‘Answer (1)
Isoelectronic means same number of electrons
CO = Number of electrons = 14
ON= 64741214
Ist excited state for H
0.53x(2)*
eee
=0.534=212A
‘Answer (4)
roxn®
I a lumber of orbit, Z = charge on nucleus
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005, Phone : 011-47623456
Solutions of Assignment (Level)
48. Answer (4)
49,
50.
51
52
53.
54,
lax,
AXeleceon = =———
ee
AXetectron = AXHe = 1.0 nm
h
an
Xie XAPhe
a)
aR,
_ ho
AAP cron 4RAP ig
AXte
[aPie = 8.0% 10% kg ms"
‘Answer (4)
Fe = 26 = 1s, 2s”, 2p, 38%, 3p®, 4s, 3%
Answer (2)
Nv = 4 = 15%, 25?
Fe® = 24 = 152, 252, 2p, 382, 3p8, 30%
Zn® = 29 = 1°, 25°, 2p8, 35°, 3p8, 4s", 30
Cu® = 28 = 18%, 25%, 2p®, 3s, 3p%, 4s°, 30"?
Answer (4)
Number of elliptical orbit in shell = (n ~ 1)
Answer (3)
h
Rasy m=tg=0.001kg, v= 100 mis
_6.6x10*kg m*s?
0.001 Kgx100 mis
bh =6.63x10- m|
Answer (1)
As the value of m= +2
ie., one value
Therefore one orbital is represented.
Answer (3)
2
ce 1|
vo Seoxr| 5-4
x [ nm
Zero unpaired
Tt T1114
‘One unpaired
Zero unpaired
Structure of Atom 7
Four unpaired
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005. Phone : 011-47623456
78 Structure of Atom Solutions of Assignment (Levelt)
0 1 2
v=3.0x10 xr09079| 5 ahi
y=3x—9 109678 em] 18 ]~3.09x10'8
40" 16
Alternatively
E=E,-E,
218x107 =. -|
E,=-2.18x10°* J
18x10" 2.1810"
(ay 16
AE = E, -E,[-0.136—(-2.18)}«10-"* = 2.0410"
E 0.13610" J
he
xe a)
vee @)
Put equation (1) in equation (2)
v= 2xae SE
he h
y= 2.041077
66x10
55. Answer (1)
= 0.309x10""8 = 3.0910 s*
Electronic configuration [Ar] 3d° represents 24 electrons
ie., [Co% =24 e]
Ni = 28-3 = 256"
Mn**
Fe =26-3=23 6
56. Answer (4)
Bohr's model explain the energy level ‘e., energy of electron in each orbital is quantized.
57. Answer (2)
58. Answer (3)
e,
aaa
Corporate Office : Aakash Tower, 8, Pusa Road, New Delhi-110005, Phone : 011-47623456
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5814)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (844)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- TY 1 Practice Paper 01A Q A 2023-04-27 2023 QDocument14 pagesTY 1 Practice Paper 01A Q A 2023-04-27 2023 QDarkAngelNo ratings yet
- CLS MED 23 24 XI Che Package 3 Level 1 Chapter 6Document26 pagesCLS MED 23 24 XI Che Package 3 Level 1 Chapter 6DarkAngelNo ratings yet
- CLS - MED 23 24 XI - Che - Package 2 - Level 2 - Chapter 4Document15 pagesCLS - MED 23 24 XI - Che - Package 2 - Level 2 - Chapter 4DarkAngelNo ratings yet
- CLS MED 23 24 XI Che Package 4 Level 2 Chapter 8Document9 pagesCLS MED 23 24 XI Che Package 4 Level 2 Chapter 8DarkAngelNo ratings yet
- Recess 2:20-2:45Document1 pageRecess 2:20-2:45DarkAngelNo ratings yet
- L-3 (Thermal Properties of Matter)Document43 pagesL-3 (Thermal Properties of Matter)DarkAngelNo ratings yet
- DPP - 3 (Thermal Properties of Matter)Document7 pagesDPP - 3 (Thermal Properties of Matter)DarkAngelNo ratings yet
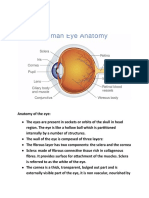
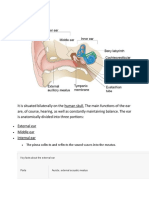
- Anatomy of The EarDocument8 pagesAnatomy of The EarDarkAngelNo ratings yet
- Added 2024Document10 pagesAdded 2024DarkAngelNo ratings yet
- Class 10 Module On Eye IDocument5 pagesClass 10 Module On Eye IDarkAngelNo ratings yet
- Anatomy of The EarDocument8 pagesAnatomy of The EarDarkAngelNo ratings yet