Professional Documents
Culture Documents
AAP January 2024 Complete Issue NeoReviews
Uploaded by
habibfmCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
AAP January 2024 Complete Issue NeoReviews
Uploaded by
habibfmCopyright:
Available Formats
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
NeoReviews
PERSPECTIVES Editor-in-Chief: Dara Brodsky, Boston, MA
Associate Editor, IOS Cases: Elizabeth Schulz, Bethesda, MD
e1 A Call for Early Detection of Cerebral Palsy Associate Editor, IOS Cases: Jayasree Nair, New York, NY
Associate Editor, CME: Santina A. Zanelli, Charlottesville, VA
Faith Kim, Nathalie Maitre, on behalf of the Cerebral Palsy Foundation Associate Editor, NeoQuest: Rita Dadiz, Rochester, NY
Associate Editor, Perspectives: Mamta Fuloria, Bronx, NY
Associate Editor, Maternal-Fetal Medicine: Brett Young, Boston, MA
Associate Editor, Video Corner: Akshaya Vachharajani, Columbia, MO
ARTICLES Assistant Editor, CME: Theodore De Beritto, Los Angeles, CA
Associate Editor, Complex Fetal Care:
e12 Nutritional Needs of the Infant with Carl Backes, Columbus, OH •
••
•••
•
•
•
Bronchopulmonary Dysplasia
Audrey N. Miller, Jennifer Curtiss, Matthew J. Kielt
EDITORIAL BOARD
Anisha Bhatia, Canton, OH MOC
•
•
•
Colby Day, Jacksonville, FL
•• •
•••
Jennifer Hanford, Columbia, MO
e25 Optimizing Nutrition in Neonates with Kidney Dysfunction Alison Chu, Los Angeles, CA
Saudamini Nesargi, Heidi Steflik, Nivedita Kamath, David Selewski, Corinne L. Leach, Buffalo, NY
Krithika Lingappan, Houston, TX
Katja M. Gist, Shina Menon Sai Mukthapuram, Cincinnati, OH
Zeynep Salih, Carmel, IN
e36 Common Clinical Scenarios of Systemic Hypertension in Thomas E. Wiswell, Honolulu, HI
Clyde Wright, Aurora, CO
the NICU
Manager, Journal Publishing: Josh Sinason
Sheema Gaffar, Rangasamy Ramanathan, Molly Crimmins Easterlin Medical Copy Editor: Beena Rao
Publisher: American Academy of Pediatrics
President: Benjamin D. Hoffman
INDEX OF SUSPICION IN THE NURSERY Chief Executive Officer/Executive Vice President:
Mark Del Monte
e50 A Term Neonate with Encephalopathy Chief Product and Services Officer/SVP Membership, Marketing,
and Publishing:
Shruthi Kumar Bharadwaj, Smriti Bhargava, Sheila Samanta Mathai, Mary Lou White
Vice President, Publishing: Mark Grimes
Jayashree Purkaystha Director, Journal Publishing: Joseph Puskarz
e53 A Newborn with Inspiratory Stridor NeoReviews™
(ISSN 1526-9906) is owned and controlled by the American Academy of Pediatrics. It is
Ishica B. Zaman, Dana Mazuru-Witten, Akshaya J. Vachharajani published monthly by the American Academy of Pediatrics, 345 Park Blvd., Itasca, IL 60143.
Statements and opinions expressed in NeoReviews™ are those of the authors and not
e56 Tachycardia in a Premature Neonate necessarily those of the American Academy of Pediatrics or its Committees. Recommendations
included in this publication do not indicate an exclusive course of treatment or serve as a
Megan Carney, Tamara Kalhan, Ellis Rochelson standard of medical care. Subscription price for NeoReviews™ for 2024: AAP/CPS Member, $160;
AAP In-Training/National A_liate Member, $145; Nonmember, $199; Nonmember In-Training /
Allied Health, $125; AAP Perinatal Section Member, $145.
Institutions call for pricing (866-843-2271).
MATERNAL-FETAL CASE STUDIES © AMERICAN ACADEMY OF PEDIATRICS, 2024. All rights reserved. Printed in USA. No part
may be duplicated or reproduced without permission of the American Academy of
Pediatrics.
e60 Fetal Injury from Maternal Penetrating Abdominal Trauma
POSTMASTER: Send address changes to NEOREVIEWS™, American Academy
in Pregnancy of Pediatrics, Member and Customer Care, 345 Park Blvd., Itasca, IL 60143.
Emily Barron, Alison Jeffries, Sarah Pelton, Katherine Vogel, Bobbi J. Byrne CME/MOC Information:
The American Academy of Pediatrics (AAP) is accredited by the Accreditation Council
for Continuing Medical Education (ACCME) to provide continuing medical education for
physicians.
The AAP designates this journal-based CME activity for a maximum of 24.0 AMA PRA
VISUAL DIAGNOSIS Category 1 Credit(s)™. Physicians should claim only the credit commensurate with
the extent of their participation in the activity.
e66 Umbilical Cord Abnormality in a Monochorionic- In order to earn CME credits and/or ABP MOC Part 2 points, you must participate in
this activity online at www.neoreviews.org, where you will view additional information,
Monoamniotic Twin Pregnancy complete your CME quizzes, and claim credit online.
Byron S. Maltez, Maryam Tarsa, Sandra L. Leibel NeoReviews™ and NeoReviewsPlus™ are supported, in part, through an educational grant
from Abbott Nutrition, a proud supporter of the American Academy of Pediatrics.
The American Academy of Pediatrics is committed to principles of equity, diversity,
and inclusion in its publishing program.
facebook.com/neoreviews
twitter.com/AAPJournals
Answer Key appears on page e65.
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
PERSPECTIVES
A Call for Early Detection of Cerebral
Palsy
Faith Kim, MD,* Nathalie Maitre, MD, PhD,† on behalf of the Cerebral Palsy Foundation
*Department of Pediatrics, Columbia University Irving Medical Center/NewYork-Presbyterian Children’s Hospital of New York, New York, NY
†
Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta, Atlanta, GA.
PRACTICE GAPS
Clinicians caring for infants who are at risk of developing cerebral palsy
(CP) should be familiar with standardized assessment tools including the
General Movements Assessment and the Hammersmith Infant Neurological AUTHOR DISCLOSURES Dr Maitre has
worked under grants from the National
Examination. Clinicians trained in these tools can use them to make an
Institutes of Health and the Cerebral
early, accurate diagnosis of CP to allow for earlier intervention and Palsy Foundation; owns a patent, care of
improved outcomes. Enlighten Mobility and Thrive
Neuromedical; and is a cofounder of
Thrive Neuromedical. Dr Kim receives
support as a principal investigator for the
OBJECTIVES After completing this article, readers should be able to: Cerebral Palsy Foundation Early Detection
Initiative and has received honoraria for
being a guest speaker, courtesy of
1. Describe the changing spectrum of cerebral palsy (CP) diagnosis in Hackensack University and Morristown
infants treated in NICUs. Medical Center. This commentary does
not contain a discussion of an
2. Describe the development and implementation of clinical guidelines for unapproved/investigative use of a
early detection of CP. commercial product/device.
3. Recognize the utility of General Movements Assessment, Hammersmith
Infant Neurological Examination, and neuroimaging in the early ABBREVIATIONS
detection of CP. AIMS Alberta Infant Motor Scale
ASD autism spectrum disorder
CP cerebral palsy
CS cramped synchronized
ABSTRACT cUS cranial ultrasound
Cerebral palsy (CP) is the most common physical disability across the DAYC Developmental Assessment of
Young Children
lifespan, but historically, CP has not been diagnosed before the age of 2
EDI Early Detection and
years. Barriers to early diagnosis ranged from lack of available biomarkers, Implementation
absence of curative treatments, perceived stigma associated with a lifelong ELGAN Extremely Low Gestational Age
Newborn Study
diagnosis, and a desire to rule out other diagnoses first. Most importantly,
FM fidgety movement
the fundamental question that remained was whether children would GMA General Movements
benefit from earlier detection and intervention given the paucity of Assessment
research. However, evidence-based guidelines published in 2017 GMFCS Gross Motor Function
Classification System
demonstrated that the General Movements Assessment, the Hammersmith HIE hypoxic-ischemic
Infant Neurological Examination, and neuroimaging can be combined with encephalopathy
other elements such as a clinical history and standardized motor HINE Hammersmith Infant
Neurological Examination
assessments to provide the highest predictive value for diagnosing CP as
HRIF high-risk infant follow-up
early as age 3 months in high-risk newborns. Implementation of these IVH intraventricular hemorrhage
guidelines has been successful in decreasing the age at CP diagnosis, MRI magnetic resonance imaging
PVL periventricular leukomalacia
particularly in high-risk infant follow-up clinics with expertise in performing
TIMP Test of Infant Motor
these assessments. Early detection of CP allows for clinical and research Performance
Vol. 25 No. 1 JANUARY 2024 e1
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
opportunities investigating earlier interventions during a critical period of neuroplasticity, with the goal of
improving developmental trajectories for children and their families. New guidelines and research are now being
developed with a focus on early, targeted interventions that continue to be studied, along with global detection
initiatives.
INTRODUCTION recent Canadian study used a prognostic bedside tool inte-
Cerebral palsy (CP) is the most common physical disabil- grating risk factors in the neonate and pregnant person
ity across the lifespan, with approximately 10,000 infants (eg, tobacco use, diabetes, preeclampsia, intra-amniotic in-
with a new diagnosis of CP every year in the United fection) and identified twice as many children with CP in
States. (1)(2)(3)(4)(5)(6) Although the prevalence of severe low-risk term infants compared with those who presented
forms of CP has declined, the overall prevalence of the dis- with neonatal encephalopathy; however, this algorithm
order has remained unchanged despite advances in obstet- has not yet been proven to be generalizable across popula-
ric and neonatal care. (7)(8)(9) Children with CP can be tions. (32)
broadly categorized into groups based on their level of risk Standardizing practices to facilitate early detection of
at birth: CP across all groups of infants based on scientific evi-
dence will optimize identification and support the develop-
1. Term infants with hypoxic-ischemic encephalopathy ment of additional effective interventions for affected
(HIE) infants. In this article, we review the definition and risk
2. Preterm infants factors for CP as well as the history of early detection, in-
3. Infants with identifiable and diverse risk factors such troducing readers to the basic elements and principles be-
as stroke, intrauterine drug exposure, cytomegalovirus, hind assessment tools being implemented in high-risk
and other infectious diseases infant programs across the world.
4. Infants with no identifiable risk factors (10)(11)(12)(13)
OVERVIEW OF CP
Among infants with moderate to severe HIE, 19% de- The definition of CP has been debated dating back to 1861
veloped CP by 2 years of age despite therapeutic hypother- when William Little first described a condition as “cerebral
mia, compared with 31% of nontreated infants. (14)(15) paresis” that started in childhood and persisted across a
(16)(17)(18)(19)(20)(21) Furthermore, it is well-established person’s lifespan. (33) The most cited definition in the lit-
that the risk of CP increases with decreasing gestational erature reflects a large international consensus and reads
age. (7)(22)(23)(24) A large systematic review demonstrated as follows: “[CP] describes a group of disorders of the develop-
a pooled prevalence of CP of 59 in 1,000 live births in in- ment of movement and posture, causing activity limitation that
fants weighing 1,000 to 1,499 g at birth and 112 in 1,000 is attributed to non-progressive disturbances that occurred in
live births in infants born at less than 28 weeks’ gestation. the developing fetal or infant brain. The motor disorders of
(9) Approximately 15% of preterm infants born between 24 [CP] are often accompanied by disturbances of sensation, cogni-
and 27 weeks’ gestation develop CP, and as more infants at tion, communication, perception, and/or behavior, and/or by a
younger gestational ages (22–23 weeks’ gestation) are sur- seizure disorder.”(34) This definition does not imply a single
viving, the rates of CP have increased to more than 20% in etiology or discrete lesion but rather describes a spectrum of
the most premature infants. (23)(25)(26)(27) However, even disease with a common phenotype but possible broad origins,
late preterm (34 to <37 weeks’ gestation) and moderately or “disturbances.”(33) Mechanisms underlying the development
preterm (32 to <34 weeks’ gestation) infants who comprise of CP are often attributed to 1) intrauterine factors (eg, placental
the majority of infants born prematurely have higher odds pathology, congenital anomalies, fetal growth restriction, drug
of developing CP compared with term infants, with white exposure), 2) peripartum events (eg, HIE, stroke, or intra-amni-
matter injury representing the predominant type of abnor- otic infection), 3) postnatal complications, most commonly due
mal neuroimaging found in this subgroup of patients. (28) to prematurity-related morbidities (eg, intraventricular hemor-
Although the American Academy of Pediatrics recom- rhage [IVH] or periventricular leukomalacia [PVL]), or 4) identi-
mends motor development surveillance at ages 9, 18, 30, fiable genetic factors. (35) However, the etiology of CP remains
and 48 months, a large proportion of term newborns who unknown in half of affected children. Recent studies have
develop CP may be undetected initially. (2)(29)(30)(31) A highlighted a more prominent role for genetic factors in the
e2 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
development of CP. Among 250 parent-offspring trios, of requiring the most support) based on self-initiated move-
which the majority (63%) were classified as having no identifi- ment; the emphasis is on sitting, walking, and wheelchair
able cause associated with CP, whole-exome sequencing identi- mobility, with the expectation that the classification remains
fied newly implicated genes in those with CP and estimated stable even into early adulthood. (44) One previous study
that 14% of cases were related to a causative genomic mutation. examining the validity of the GMFCS tool in children with
(36) A meta-analysis that included exome or genome sequenc- CP ranging from 16 months to 13 years old found that chil-
ing in individuals with CP found an overall diagnostic yield of dren younger than 6 years who were initially classified as
8% to 16% in those with risk factors for CP versus 14% to level II to IV were more likely to be reclassified, often to a
48% in those without risk factors; this finding is similar in pa- lower functional level when they were older compared with
tients with an intellectual disability or autism spectrum disorder children who were first classified as level I or V. (45) This
(ASD). (37) Thus, a genetic assessment is now included in a was particularly true in children younger than 4 years, indi-
systematic approach to an evaluation of CP. cating less reliability of this tool in predicting functional
The definition of CP acknowledges the complexity of abilities later in life for younger children. (45) Although the
this disease beyond that of a physical disability. Almost GMFCS now contains a descriptive category for infants
75% of children with CP experience another comorbidity, with CP aged 0 to 2 years, it should be used with caution
which may worsen the impact of the disorder on func- as a valid indicator of functional abilities in early childhood.
tional limitations and quality of life. (10)(38)(39) A meta- In one small study including 77 children with CP younger
analysis using data from CP registers found that most than 2 years, 42% required reclassification by the age of 4
children with CP experienced pain, 1 in 2 had an intellec- years—two-thirds of them were reclassified to a better func-
tual disability, 1 in 3 could not walk or had a hip displace- tional GMFCS level. (46)
ment, and 1 in 4 could not talk; epilepsy and other Description of CP type and distribution in the first 2
neurosensory impairments were common. (39) Children years can be challenging, especially for preterm infants.
with CP are also at high risk of developing behavioral dis- The classification algorithms developed by Dr Kuban for
orders. One study found that 7% of children with CP had the Extremely Low Gestational Age Newborn Study (EL-
ASD compared with 1% of the general population; ASD GAN) study are helpful for neonatologists as they acknowl-
was most prevalent in children with nonspastic forms of edge the difficulties in tone assessment resulting from
CP. (6) The added burdens of comorbidities are further prolonged medical stays and interrupted development.
unevenly distributed amongst different subtypes of CP, of- (47) In general, hemiplegia involving 1 side of the body,
ten lesser in those with spastic hemiplegic or diplegic var- and diplegia involving bilateral lower limbs are the most
iants. (39) Despite CP being a life-long disability, almost common subtypes of CP affecting more than 75% of chil-
all affected children are expected to have a normal life ex- dren; they are associated with lower GMFCS levels. (48)
pectancy. The lowest survival rates are reported in those Quadriplegia involves all 4 limbs and can manifest as
with more severe disabilities, particularly if they have spasticity with more severe functional limitations. (48)
other disorders such as epilepsy, severe cognitive disabil- There are 3 main types of abnormal tone including spastic
ity, or gastrostomy-tube dependence. (40)(41)(42) (87%), dyskinetic (7.5%), and ataxic (4%) CP but different
CP can be conceptualized in different frameworks, but types can coexist and are difficult to differentiate in the
the most globally accepted is the World Health Organiza- first 2 years of age. (7)(49) Spastic CP often manifests af-
tion’s International Classification of Function, which places ter damage to the periventricular white matter, (44) dyski-
disability and functioning as outcomes of interactions be- netic CP after damage to the subcortical gray matter (eg,
tween health conditions and contextual factors. (43) Evalua- basal ganglia and thalamus), and ataxic CP is most often
tion of CP then encompasses medical, individual, social, due to cerebellar injury or malformations. (49)
and environmental factors, which all contribute to health
and activity. Neonatologists are most familiar with the HISTORY BEHIND EARLY DETECTION
Gross Motor Function Classification System (GMFCS), ini- Historically, CP was thought of as an “unseen handicap,”
tially developed and validated for patients with CP aged 2 to not diagnosed due to the emerging nature of voluntary
12 years, but sometimes used in neonatal research studies movements in infancy and the delayed evolution of abnor-
for children without CP. (44) The GMFCS categorizes chil- mal tone. (50) The notion that CP was preceded by a neu-
dren with CP into 1 of 5 levels (levels I–V depending on rologically silent period prevailed, and clinicians adopted a
support needed for ambulation and mobility, with level V wait-and-see approach until recently, when the advent of
Vol. 25 No. 1 JANUARY 2024 e3
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
diagnostic tools and international consensus backed by healthy infants. (53) FMs are small, circular, and multiplanar
mounting evidence has favored early detection. rapid and continuous movements that involve all parts of
the body including the extremities, head, neck, and trunk.
BASIC PRINCIPLES AND EVIDENCE OF TOOLS The presence of FMs represents a normal finding whereas the
USED FOR EARLY DETECTION absence of FMs at 3 to 4 months’ corrected age is highly
In 2017, a set of clinical guidelines based on systematic re- predictive of CP. (53)
views and evidence-based guidelines was published, which Given the need to rely on gestalt or perception of move-
delineated an algorithm that used a combination of vali- ment pattern quality, the GMA involves short video re-
dated tools to provide an early, accurate diagnosis of CP as cordings lasting 3 to 5 minutes that are then interpreted
early as 3 months in specific cases. (51) The guidelines in- after filming at 2 different time points: at term postmenst-
corporate several high-evidence assessment tools (the Gen- rual age during the writhing period and 3 to 4 months’
eral Movements Assessment [GMA], the Hammersmith corrected age during the fidgety period. Because the GMA
Infant Neurological Examination [HINE], the Developmen- has become a standardized qualitative tool with high inter-
tal Assessment of Young Children (DAYC)-2, and brain observer reliability and validity, clinicians can become cer-
magnetic resonance imaging [MRI]) that measure differ- tified as GMA basic and/or advanced readers after taking
ent but complementary constructs in addition to a clinical a 31=2-day course offered by the General Movements Trust.
history and other standardized motor assessments. (51) (54)(55)
The first study describing the predictive value of the
The GMA GMA was published by Dr. Prechtl when he evaluated gen-
General movements are part of a spontaneous motor rep- eral movements during the writhing and fidgety periods in
ertoire that are endogenously generated and are present 130 preterm and term infants with both low-risk cranial ul-
from fetal life until about 5 months’ corrected age. In the trasound (cUS) findings (eg, grade 1 IVH) and high-risk
early 1990s, Dr. Prechtl first described the GMA, a novel cUS deficits (eg, grade 2–4 IVH, PVL) and examined their
visual approach to characterize an infant’s pattern of spon- neurologic outcomes by age 2 years. (55) FMs had a higher
taneous movements. (52) He observed that the quality of sensitivity and specificity of 95% and 96%, respectively, in
these movements were altered in both preterm and term predicting neurologic outcomes compared with cUS, which
infants who had an underlying brain injury compared had a sensitivity and specificity of 80% and 83%, respec-
with healthy controls. (52) He described 2 distinct general tively. (55) Most infants with CS movements during the
movement patterns that comprise the GMA and can be writhing period developed absent FMs, and all but 1 infant
seen in both term and preterm infants: 1) writhing move- (43/44) with absent FMs developed CP. (55) Several studies
ments and 2) fidgety movements (FMs). (53) During the replicated these findings, demonstrating that trajectories of
writhing period, which begins around 36 weeks’ postmenst- CS movements followed by absent FMs were highly predic-
rual age and persists until 9 weeks’ corrected age, general tive of CP. (53)(54)(55)(56)(57)(58)(59) A systematic review
movements are categorized as normal writhing, poor reper- of 19 studies on high-risk populations including preterm
toire, chaotic, or cramped synchronized (CS). (53) Normal and low-birthweight infants demonstrated a pooled sensitiv-
writhing movements involve the whole body in a variable, ity of 98% and specificity of 91% of the GMA for CP, most
complex sequence with fluent movements that start and predictive during the fidgety period around 3 to 4 months’
end gradually, occurring frequently while the infant is corrected age, which was better than the use of cUS or
awake or sleeping with no external stimuli whereas poor term-postmenstrual age brain MRI. (60)
repertoire movements are more monotonous in nature. (53)
The most significant abnormal general movements to rec- The HINE
ognize during this period are CS patterns, which are char- The HINE is a scorable neurologic examination consisting
acterized by synchronous contractions of all limbs followed of 26 items that assess cranial nerves, tone, posture,
by a relaxation phase and are highly predictive of the devel- movements, reflexes, and reactions. It can be administered
opment of spastic CP, most notably if they are present at in infants between 2 months and 2 years of age. (61) It
term-postmenstrual age. (53) At about 7 to 8 weeks’ cor- was first described in 1999 by Dr Haataja and validated
rected age, FMs start to emerge and replace writhing move- for use in a cohort of 119 healthy term infants followed
ments, persisting until 5 months’ corrected age when they until age 18 months, providing a distribution of scores
are then replaced by goal-directed, voluntary movements in across each domain. (62) It allows trained examiners to
e4 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
derive global optimality scores ranging from 0 to 78 and a standardized assessment tool that evaluates development
separate asymmetry score. It can be performed in 5 to 10 mi- from birth through age 5 years using an interactive ques-
nutes and has good interobserver reliability after training. (62) tionnaire for parents that can also be administered over
Longitudinal assessments allow examiners to differentiate be- telehealth. The physical development domain in the most
tween transient versus permanent abnormalities. The HINE updated version measures both gross motor and fine mo-
can provide both diagnostic and prognostic information in tor skills based on parental report, direct observation, or
high-risk infants. In the largest single study to date of 1,541 in- assessment and takes about 10 minutes to administer
fants discharged from the NICU, Romeo et al performed the with good reliability reported. (73)(74) Although the
HINE at different time points during the first 2 years of age AIMS, TIMP, and DAYC do not require highly special-
and reported cutoff scores for each age window from 3 to 12 ized training and/or additional resources compared to
months that had high prognostic value for later CP diagnoses. the GMA, clinicians should not use any tool in isolation
(63) In those who had documented brain insults, HINE scores for the purpose of predicting or diagnosing CP; rather
could assist with severity prognostication. (63) these tools should be used to help identify at-risk infants
Since then, numerous studies have evaluated the HINE’s and follow the trajectory of motor development while
predictive value in various populations. (61)(64)(65)(66)(67) screening those who may require closer surveillance.
Romeo et al recently demonstrated that 50% of low-risk (51)(75)
preterm infants with a HINE score less than 73 at 12
months’ corrected age had normal neurologic outcomes by Neuroimaging
age 2 years, thus providing more appropriate cutoff scores Although neuroimaging is helpful in early identification
for these patients. (68) The HINE may help assess the se- of CP, 10% to 15% of infants with CP have normal neuro-
verity of CP at 2 years of age in infants with documented imaging; therefore, similar to the other assessment tools
brain insults, and combinations of global and asymmetry mentioned in this review, it should not be used in isola-
scores on the HINE may help categorize hemiplegic CP. tion when making or excluding a diagnosis of CP. (51)(76)
(61)(69)(70) In a systematic review with over 2,400 infants and a CP
prevalence of 9.4%, sequential cUS in the NICU had a
Other Standardized Motor Assessments pooled sensitivity of 74% (95% confidence interval [CI],
Several standardized motor assessments are available, which 63%–83%) and specificity of 92% (95% CI, 81%–96%) to
can be performed in both preterm and term infants and predict CP in both preterm and high-risk term infants, es-
have been studied for their predictive value for CP, namely pecially in cases of grade 3 or 4 IVH, cerebellar injury,
the Alberta Infant Motor Scale (AIMS), the Test of Infant and cystic PVL. (60)(77)(78)(79) Periventricular hemor-
Motor Performance (TIMP), and the DAYC. The AIMS is a rhagic infarction, a more accurate term for grade 4 IVH,
58-item standardized tool that evaluates gross motor develop- confers a high risk for CP, and white matter injury confers
ment from birth until 18 months of age or when indepen- a 20-fold increased odds of developing CP. (80)(81)
dent ambulation is achieved and requires direct observation Evidence to support the routine use of MRI in preterm
of the infant in prone, supine, sitting, and standing posi- infants to predict CP is mixed, with poor sensitivity
tions. (71) The TIMP is a 42-item standardized tool that can (65%–71%) and good to excellent specificity (84%–95%).
be performed as early as 34 weeks’ gestational age until 4 (81)(82)(83) The Choosing Wisely campaign endorsed by
months’ corrected gestational age and requires both direct the American Academy of Pediatrics recommends avoid-
observation and items elicited such as cranial nerve function ing routine screening MRIs at term-equivalent age in pre-
and evaluation of antigravity control. (72) Both evaluations term infants given the lack of data to suggest improved
can take from 20 to 30 minutes to administer, and the prediction of long-term outcomes; however, MRI should
AIMS can be administered over telehealth but the TIMP be considered in infants with HIE, stroke, brain malfor-
should be conducted in person. The TIMP should be admin- mations, PVL, or severe IVH. (84) Notably, the Kidokoro
istered by clinicians such as physical and occupational thera- scoring (MRI scoring system of abnormalities that is spe-
pists in the NICU or early intervention programs who have cific for preterm infants with IVH) performed in preterm
undergone training through workshops and online modules; infants at term-equivalent age is used in Australia and pro-
however, the AIMS has been shown to have good reliability vides strong evidence of predicting outcomes accurately.
between both experienced and novice examiners because (85) Newer imaging techniques and modalities may allow
it relies on direct observation. (71) The DAYC is a more accurate prognostication of diagnosis, topography,
Vol. 25 No. 1 JANUARY 2024 e5
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
and severity in the future. (86) Certainly, if CP is suspected, and cytomegalovirus infection, should have a GMA, HINE,
clinicians should consider MRI as part of the diagnostic thorough medical history conducted including review of
evaluation in a term infant without risk factors, for cortical any detectable risk factors for CP or parent-identified con-
or vascular malformations among possible etiologies. (87) cerns, review of imaging, and a standardized motor assess-
ment performed starting at 3 to 4 months’ corrected age; in
Evidence to Support the Combination of Multiple infants older than 5 months, the HINE, MRI, and standard-
Tools ized motor assessments are recommended. (12)(13)(51)
None of the assessment tools described herein (GMA,
HINE, standardized motor assessments, and neuroimag- IMPLEMENTATION OF CLINICAL GUIDELINES
ing) should be used in isolation for detection of CP as
Because of the inconsistent ways in which neurologic ex-
they each offer a complementary construct:
aminations were performed and documented in high-risk
1. The GMA evaluates the quality of spontaneous move- infant follow-up (HRIF) clinics, the neonatal neurodeve-
ments and provides information on neural function and lopmental team at Nationwide Children’s Hospital, which
integrity. has over 5,000 yearly visits, was trained in the HINE with
2. The HINE is a standardized neurologic examination. the help of a Hammersmith trained neurologist and by de-
3. Standardized motor assessments (eg, AIMS, TIMP, and veloping a preparation course work and on-site practice.
physical domain of the DAYC) are tools to evaluate fine (89) Following the implementation of the HINE, which
and gross motor impairment. was administered at the 3- to 4-month, 9- to 12-month,
4. Neuroimaging (eg, brain MRI or cUS) detects brain ab- and 22- to 26-month visits up until 2 years’ corrected age,
normalities or injury associated with CP. the age at diagnosis of CP was reduced from 28 months
to 15.7 months without an increase in the number of new
Serial HINEs between 3 and 24 months’ corrected age diagnoses while maintaining adequate inter-rater reliabil-
and the GMA at 3 to 4 months’ corrected age in infants ity. (89) By successfully learning and implementing the
with newborn-detectable risk factors have shown promise HINE and the GMA into clinical practice at a single site
in predicting CP, especially the combination of absent while standardizing documentation in the electronic medi-
FMs and HINE score of less than 50. (88) The pooled pre- cal record, the team was able to demonstrate a reduction
dictive power of HINE, GMA, and cUS or MRI in high- in age at CP diagnosis, demonstrating the feasibility of us-
risk term and preterm infants has shown sensitivity, spe- ing this tool in clinical practice. (90)
cificity, and positive and negative predictive values greater Following the publication of the clinical guidelines for
than 98% in predicting the development of CP by 2 years
early detection in 2017, single centers started implement-
compared to each tool alone. (76) The caveat in this study,
ing these recommendations, reducing the age at diagnosis
however, was that HINE examiners, GMA readers, and
to 8.5 months in one cohort with 98% of those diagnosed
neuroimaging readers were all experts in the field of CP.
with CP being referred to CP-specific early intervention
programs. (89)(91)(92) The largest implementation study
Use of CP Early Detection Tools in Neonatal
to date included the formation of an Early Detection and
Follow-Up
Implementation (EDI) Network composed of 5 diverse US-
The international clinical practice guidelines published in
based HRIF clinics. The network reduced the age at CP di-
2017 were developed by a multidisciplinary panel of scien-
agnosis using quality improvement methodology and im-
tific and clinical experts in CP and parent stakeholders,
and outlined 12 recommendations based on best evidence- plementation science on a large scale by transitioning to
based assessments with an algorithm for early detection of an earlier visit at 3 to 4 months and incorporating the
CP (Fig). (51) Early detection is feasible and accurate and GMA, HINE, medical history, and standardized motor
can lead to earlier CP-specific interventions whereas an in- function assessment. (93) Within 1 year, the network low-
terim designation of “high risk of CP” should be used if ered the age at diagnosis from 19.5 months’ corrected age
the clinician is concerned but cannot be certain of the di- to 9.5 months’ corrected age with no increase in the num-
agnosis. (51) In general, infants younger than 5 months' ber of CP diagnoses. (93) Recent publications show that
corrected age who have identifiable risks such as the neu- they sustained their change 5 years later, even through the
ral insults previously mentioned, but also others such as addition of new sites and the challenges related to the
intrauterine growth restriction, intrauterine drug exposure, COVID-19 pandemic. (94)
e6 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
Figure. Pathway to early detection. A represents the best available evidence pathway per the international clinical guidelines published by Novak et al
in 201751; B is next best if some tools in A are not available. AIMS5Alberta Infant Motor Scale, CP5cerebral palsy, DAYC5Developmental Assessment of
Young Children, GMs5Prechtl Qualitative Assessment of General Movements, HINE5Hammersmith Infant Neurological Examination, IUGR5intrauterine
growth restriction, MAI5Motor Assessment of Infants, NSMDA5Neuro-Sensory Motor Development Assessment, TIMP5Test of Infant Motor Perfor-
mance. (Modified from “Early Recognition of Cerebral Palsy: A Pathway to Referral” published online in 2018. Reprinted with permission from the Cerebral
Palsy Foundation in New York, NY.)
CAREGIVER PERCEPTION OF EARLY DIAGNOSIS there is a missing diagnostic component or a negative result
Population data support the notion that delaying conversations (Table). (94)(97) During a focus group involving caregivers
surrounding even the suspicion of CP can be detrimental to of children with CP describing their experiences surround-
parental well-being. The vast majority of caregivers already ing CP diagnosis and the concept of using a designation
suspect the diagnosis before being told, and in 1 study, more early on, parents expressed that a designation was an accept-
than 40% of caregivers experienced dissatisfaction and resent- able alternative to start these conversations and could be re-
ment about a delayed diagnosis, which correlated with later visited even if a diagnosis was ultimately not made. (98)
depression. (95) On a large scale, the US-based implementa- Globally, clinicians who have shifted their practice toward
tion network found that 90% of parents reported receiving early detection of CP have adopted this designation to pro-
empathy and support at the diagnosis visit. (95) This positive vide a framework for shared decision-making and establish-
perception of early CP diagnosis has been confirmed, with pa- ing a common language between families and high-risk
rents generally wanting more information on the next steps. follow-up clinicians. Importantly, this shift to early detection
(81)(96)(97) Parents of children with CP have stated that ear- has allowed for the study of earlier interventions in children
lier diagnosis or use of a high-risk designation was beneficial with CP even younger than 2 years. The results are promis-
to their family and their child and was a priority in an honest ing, and an increasing pipeline of studies testing new inter-
yet positive conversation with diagnosis-related education and ventional approaches is actively underway. (99)(100)
resources. (97)
To address starting conversations earlier, a consensus CONCLUSIONS
statement was put forth by the EDI network and the Cana- Across NICUs and HRIF clinics around the world, a shift
dian Neonatal Follow-Up Network in 2022 to adopt a “high- toward early detection of CP to drive new early interven-
risk for CP” designation when a diagnosis is suspected but tions has evolved from decades of historical challenges
Vol. 25 No. 1 JANUARY 2024 e7
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
Table. Proposed criteria for diagnosis of cerebral palsy (CP) versus high-risk clinical designation.
Basic elements of diagnosis of CP Clinical history consistent with the etiology of CP
Neurologic exam with evidence of impairment
Decrease in motor function on standardized motor assessment
Neuroimaging findings associated with CP (eg, MRI or cUS with evidence of stroke,
HIE, grade 3–4 IVH, hydrocephalus, periventricular leukomalacia)
Positive biomarkers for CP:
! Genetic condition associated with CP
! No underlying progressive disorder
! Hammersmith Infant Neurological Exam scores below threshold for age
! Cramped synchronized and/or absent fidgety movements on the General
Movements Assessment
High risk for CP clinical designation A basic element is missing/an assessment is not performed
Basic element with negative results for predicting later CP but others are positive
First evaluation of child before age of 2 years with clinical concern but no previous
evaluations have demonstrated clear concerns
Adapted from Maitre NL, Byrne R, Duncan A, et al; CP EDI Consensus Group; Canadian Neonatal Follow-up Network. “High-risk for cerebral palsy”
designation: A clinical consensus statement. J Pediatr Rehabil Med. 2022;15(1):165–174.
and advances. The feasibility of decreasing the age at CP diag- 2. Noritz G, Davidson L, Steingass K; Council on Children with
Disabilities, American Academy for Cerebral Palsy and
nosis on a large scale has been proven, starting with practices
Developmental Medicine; Council on Children with Disabilities,
in the NICU and followed by an HRIF visit at 3 to 4 months’ American Academy for Cerebral Palsy and Developmental Medicine.
corrected age. (84) Early diagnosis and high-risk designation Providing a primary care medical home for children and youth with
are well-accepted by caregivers, and these conversations can cerebral palsy. Pediatrics. 2022;150:e2022060055
and should start early, even while in the NICU when a fu- 3. Herskind A, Greisen G, Nielsen JB. Early identification and
intervention in cerebral palsy. Dev Med Child Neurol. 2015;57(1):29–36
ture diagnosis of CP is suspected. Through the implementa-
4. Maenner MJ, Blumberg SJ, Kogan MD, Christensen D, Yeargin-
tion of evidence-based international clinical guidelines, after Allsopp M, Schieve LA. Prevalence of cerebral palsy and intellectual
receiving training in the use of these new assessment tools, disability among children identified in two U.S. National Surveys,
we can optimize the developmental trajectories of children 2011-2013. Ann Epidemiol. 2016;26(3):222–226
with CP. We can refer these children earlier to interventions 5. McIntyre S, Goldsmith S, Webb A, et al; Global CP Prevalence
Group. Global prevalence of cerebral palsy: a systematic analysis.
during a critical period of neuroplasticity to reduce motor
Dev Med Child Neurol. 2022;64(12):1494–1506
deficits and secondary impairments. All this can happen
6. Christensen D, Van Naarden Braun K, Doernberg NS, et al. Prevalence
while promoting better support, education, and well-being of cerebral palsy, co-occurring autism spectrum disorders, and motor
for former NICU families—a goal that has always been cen- functioning: Autism and Developmental Disabilities Monitoring
tral to NICU follow-up programs. Network, USA, 2008. Dev Med Child Neurol. 2014;56(1):59–65
7. Sellier E, Platt MJ, Andersen GL, Kr€ageloh-Mann I, De La Cruz J,
Cans C; Surveillance of Cerebral Palsy Network. Decreasing
prevalence in cerebral palsy: a multi-site European population-based
American Board of Pediatrics study, 1980 to 2003. Dev Med Child Neurol. 2016;58(1):85–92
Neonatal-Perinatal Content 8. Van Naarden Braun K, Doernberg N, Schieve L, Christensen D,
Specifications Goodman A, Yeargin-Allsopp M. Birth prevalence of cerebral palsy:
a population-based study. Pediatrics. 2016;137(1):1–9
• Know the evolution of neurodevelopmental
9. Oskoui M, Coutinho F, Dykeman J, Jett"e N, Pringsheim T. An
impairments during development and the update on the prevalence of cerebral palsy: a systematic review and
difference between transient and permanent meta-analysis. Dev Med Child Neurol. 2013;55(6):509–519
impairments in NICU graduates (eg, 10. McIntyre S, Morgan C, Walker K, Novak I. Cerebral palsy–don’t
developmental delay vs. intellectual disability; delay. Dev Disabil Res Rev. 2011;17(2):114–129
tone abnormalities vs. cerebral palsy). 11. McIntyre S, Taitz D, Keogh J, Goldsmith S, Badawi N, Blair E. A
systematic review of risk factors for cerebral palsy in children born
• Know the significance of persistent neuromotor at term in developed countries [review]. Dev Med Child Neurol.
abnormalities in infancy (including asymmetries). 2013;55(6):499–508
12. Ong LT, Fan SWD. The association between congenital
cytomegalovirus infection and cerebral palsy: A systematic review
and meta-analysis. J Paediatr Child Health. 2022;58(12):2156–2162
References 13. Benninger KL, Purnell J, Conroy S, et al; NCH Early Developmental
1. Paneth N, Hong T, Korzeniewski S. The descriptive epidemiology of Group. Intrauterine drug exposure as a risk factor for cerebral palsy.
cerebral palsy. Clin Perinatol. 2006;33(2):251–267 Dev Med Child Neurol. 2022;64(4):453–461
e8 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
14. Badawi N, Felix JF, Kurinczuk JJ, et al. Cerebral palsy following 31. Lipkin PH, Macias MM; Council on Children with Disabilities,
term newborn encephalopathy: a population-based study. Dev Med Section on Developmental and Behavioral Pediatrics. Promoting
Child Neurol. 2005;47(5):293–298 optimal development: identifying infants and young children with
15. Aslam S, Strickland T, Molloy EJ. Neonatal encephalopathy: need for developmental disorders through developmental surveillance and
recognition of nultiple etiologies for optimal management. Front screening. Pediatrics. 2020;145(1):E20193449
Pediatr. 2019;7:142 32. Rouabhi A, Husein N, Dewey D, et al; Canadian Cerebral Palsy
16. Pappas A, Milano G, Chalak LF. Hypoxic-ischemic encephalopathy: Registry. Development of a bedside tool to predict the diagnosis of
changing outcomes across the spectrum. Clin Perinatol. 2023; cerebral palsy in term-born neonates. JAMA Pediatr. 2023;177(2):
50(1):31–52 177–186
17. Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. 33. Bax M, Goldstein M, Rosenbaum P, et al; Executive Committee for
Hypothermia for neonatal hypoxic ischemic encephalopathy: an the Definition of Cerebral Palsy. Proposed definition and
updated systematic review and meta-analysis. Arch Pediatr Adolesc classification of cerebral palsy, April 2005. Dev Med Child Neurol.
Med. 2012;166(6):558–566 2005;47(8):571–576
18. Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling 34. Bax MC. Terminology and classification of cerebral palsy. Dev Med
with mild systemic hypothermia after neonatal encephalopathy: Child Neurol. 1964;6:295–297
multicentre randomised trial. Lancet. 2005;365(9460):663–670 35. Stavsky M, Mor O, Mastrolia SA, Greenbaum S, Than NG, Erez O.
19. Simbruner G, Mittal RA, Rohlmann F, Muche R; Cerebral palsy: trends in epidemiology and recent development in
neo.nEURO.network Trial Participants. Systemic hypothermia after prenatal mechanisms of disease, treatment, and prevention. Front
neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatr. 2017;5:21
Pediatrics. 2010;126(4):e771–e778 36. Jin SC, Lewis SA, Bakhtiari S, et al. Mutations disrupting
20. Shankaran S, Natarajan G, Chalak L, Pappas A, McDonald SA, neuritogenesis genes confer risk for cerebral palsy. Nat Genet.
Laptook AR. Hypothermia for neonatal hypoxic-ischemic 2020;52(10):1046–1056
encephalopathy: NICHD Neonatal Research Network contribution to 37. Gonzalez-Mantilla PJ, Hu Y, Myers SM, et al. Diagnostic yield of
the field. Semin Perinatol. 2016;40(6):385–390 exome sequencing in cerebral palsy and implications for genetic
21. Garfinkle J, Li P, Boychuck Z, Bussi#eres A, Majnemer A. Early testing guidelines: a systematic review and meta-analysis. JAMA
Clinical features of cerebral palsy in children without perinatal risk Pediatr. 2023;177(5):472–478
factors: a scoping review. Pediatr Neurol. 2020;102:56–61 38. Shaunak M, Kelly VB. Cerebral palsy in under 25 s: assessment and
22. Shankaran S, Laptook AR, Ehrenkranz RA, et al; National Institute management (NICE Guideline NG62). Arch Dis Child Educ Pract Ed.
of Child Health and Human Development Neonatal Research 2018;103(4):189–193
Network. Whole-body hypothermia for neonates with hypoxic- 39. Novak I, Hines M, Goldsmith S, Barclay R. Clinical prognostic
ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584 messages from a systematic review on cerebral palsy. Pediatrics.
23. Natarajan G, Pappas A, Shankaran S. Outcomes in childhood 2012;130(5):e1285–e1312
following therapeutic hypothermia for neonatal hypoxic-ischemic 40. Shevell MI, Dagenais L, Hall N; REPACQ Consortium. The
encephalopathy (HIE). Semin Perinatol. 2016;40(8):549–555 relationship of cerebral palsy subtype and functional motor
24. Beaino G, Khoshnood B, Kaminski M, et al; EPIPAGE Study Group. impairment: a population-based study—CP subtype and functional
Predictors of cerebral palsy in very preterm infants: the EPIPAGE motor impairment. Dev Med Child Neurol. 2009;51(11):872–877
prospective population-based cohort study. Dev Med Child Neurol. 41. Westbom L, Bergstrand L, Wagner P, Nordmark E. Survival at 19
2010;52(6):e119–e125 years of age in a total population of children and young people with
25. Himpens E, Van den Broeck C, Oostra A, Calders P, cerebral palsy. Dev Med Child Neurol. 2011;53(9):808–814
Vanhaesebrouck P. Prevalence, type, distribution, and severity of 42. Hutton JL, Pharoah PO. Life expectancy in severe cerebral palsy.
cerebral palsy in relation to gestational age: a meta-analytic review. Arch Dis Child. 2006;91(3):254–258
Dev Med Child Neurol. 2008;50(5):334–340 43. World Health Organization. International Classification of
26. O’Shea TM, Allred EN, Dammann O, et al; ELGAN study Functioning, Disability and Health: ICF. Geneva, Switzerland:
Investigators. The ELGAN study of the brain and related disorders World Health Organization; 2001. https://apps.who.int/iris/handle/
in extremely low gestational age newborns. Early Hum Dev. 10665/42407 Accessed October 12, 2023
2009;85(11):719–725 44. Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B.
27. Ishii N, Kono Y, Yonemoto N, Kusuda S, Fujimura M; Neonatal Development and reliability of a system to classify gross motor
Research Network, Japan. Outcomes of infants born at 22 and 23 function in children with cerebral palsy. Dev Med Child Neurol.
weeks’ gestation. Pediatrics. 2013;132(1):62–71 1997;39(4):214–223
28. Smyrni N, Koutsaki M, Petra M, et al. Moderately and late preterm 45. Palisano RJ, Cameron D, Rosenbaum PL, Walter SD, Russell D.
infants: Short- and long-term outcomes from a registry-based cohort. Stability of the gross motor function classification system. Dev Med
Front Neurol. 2021;12:628066 Child Neurol. 2006;48(6):424–428
29. Boychuck Z, Andersen J, Bussi#eres A, et al; Prompt Group. 46. Gorter JW, Ketelaar M, Rosenbaum P, Helders PJ, Palisano R. Use
International expert recommendations of clinical features to prompt of the GMFCS in infants with CP: the need for reclassification at
referral for diagnostic assessment of cerebral palsy. Dev Med Child age 2 years or older. Dev Med Child Neurol. 2009;51(1):46–52
Neurol. 2020;62(1):89–96 47. Kuban KC, Allred EN, O’Shea M, Paneth N, Pagano M, Leviton A;
30. Noritz GH, Murphy NA; Neuromotor Screening Expert Panel. ELGAN Study Cerebral Palsy-Algorithm Group. An algorithm for
Motor delays: early identification and evaluation. Pediatrics. identifying and classifying cerebral palsy in young children. J
2013;131(6):e2016–e2027 Pediatr. 2008;153(4):466–472
Vol. 25 No. 1 JANUARY 2024 e9
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
48. Novak I. Evidence-based diagnosis, health care, and rehabilitation for based on healthy, term infants age 3-7 months. J Pediatr. 2022;
children with cerebral palsy. J Child Neurol. 2014;29(8):1141–1156 244:79–85.e12
49. Zhou J, Butler EE, Rose J. Neurologic correlates of gait 66.Pizzardi A, Romeo DMM, Cioni M, Romeo MG, Guzzetta A. Infant
abnormalities in cerebral palsy: implications for treatment. Front neurological examination from 3 to 12 months: predictive value of
Hum Neurosci. 2017;11:103 the single items. Neuropediatrics. 2008;39(6):344–346
50. Velde AT, Morgan C, Novak I, Tantsis E, Badawi N. Early diagnosis 67. Romeo DM, Bompard S, Serrao F, et al. Early neurological
and classification of cerebral palsy: an historical perspective and assessment in infants with hypoxic ischemic encephalopathy treated
barriers to an early diagnosis. J Clin Med. 2019;8(10):1599 with therapeutic hypothermia. J Clin Med. 2019;8(8):1247
51. Novak I, Morgan C, Adde L, et al. Early, accurate diagnosis and early 68. Romeo DM, Apicella M, Velli C, et al. Hammersmith Infant
intervention in cerebral palsy: advances in diagnosis and treatment. Neurological Examination in low-risk infants born very preterm: a
JAMA Pediatr. 2017;171(9):897–907 longitudinal prospective study. Dev Med Child Neurol. 2022;
52. Prechtl HF, Ferrari F, Cioni G. Predictive value of general 64(7):863–870
movements in asphyxiated fullterm infants. Early Hum Dev. 69.Romeo DMM, Cioni M, Scoto M, Mazzone L, Palermo F, Romeo
1993;35(2):91–120 MG. Neuromotor development in infants with cerebral palsy
53. Einspieler C, Prechtl HFR. Prechtl’s assessment of general investigated by the Hammersmith Infant Neurological Examination
movements: a diagnostic tool for the functional assessment of the during the first year of age. Eur J Paediatr Neurol. 2008;12(1):24–31
young nervous system. Ment Retard Dev Disabil Res Rev. 2005; 70. Hay K, Nelin M, Carey H, Chorna O, Moore-Clingenpeel Ma Mas
11(1):61–67 M, Maitre N; NCH Early Developmental Group. Hammersmith
54. Kwong AKL, Fitzgerald TL, Doyle LW, Cheong JLY, Spittle AJ. Infant Neurological Examination asymmetry score distinguishes
Predictive validity of spontaneous early infant movement for later hemiplegic cerebral palsy from typical development. Pediatr Neurol.
cerebral palsy: a systematic review. Dev Med Child Neurol. 2018;87:70–74
2018;60(5):480–489 71. Eliks M, Gajewska E. The Alberta Infant Motor Scale: A tool for the
55. Prechtl HF, Einspieler C, Cioni G, Bos AF, Ferrari F, Sontheimer D. assessment of motor aspects of neurodevelopment in infancy and
An early marker for neurological deficits after perinatal brain early childhood. Front Neurol. 2022;13:927502
lesions. Lancet. 1997;349(9062):1361–1363 72. Campbell SK, Wright BD, Linacre JM. Development of a functional
56. Porro M, Fontana C, Giann#ı ML, et al. Early detection of general movement scale for infants. J Appl Meas. 2002;3(2):190–204
movements trajectories in very low birth weight infants. Sci Rep. 73. Voress J, Maddox T. Developmental Assessment of Young Children.
2020;10(1):13290 Austin, TX: PRO-ED; 1998
57. Einspieler C, Marschik PB, Bos AF, Ferrari F, Cioni G, Prechtl HF. 74. Swartzmiller MD. Test review: Developmental Assessment of Young
Early markers for cerebral palsy: insights from the assessment of Children-Second Edition (DAYC-2). J Psychoed Assess. 2014;32:577–580
general movements. Future Neurol. 2012;7:709–717 doi: 10.2217/ 75. Maitre NL, Slaughter JC, Aschner JL. Early prediction of cerebral
fnl.12.60 palsy after neonatal intensive care using motor development
58. Morgan C, Crowle C, Goyen TA, et al. Sensitivity and specificity of trajectories in infancy. Early Hum Dev. 2013;89(10):781–786
General Movements Assessment for diagnostic accuracy of detecting 76. Morgan C, Romeo DM, Chorna O, et al. The pooled diagnostic
cerebral palsy early in an Australian context. J Paediatr Child Health. accuracy of neuroimaging, general movements, and neurological
2016;52(1):54–59 examination for diagnosing cerebral palsy early in high-risk infants:
59. Støen R, Boswell L, de Regnier RA, et al. The predictive accuracy of a case control study. J Clin Med. 2019;8(11):1879
the General Movement Assessment for cerebral palsy: a prospective, 77. Hand IL, Shellhaas RA, Milla SS; Committee on Fetus and
observational study of high-risk infants in a clinical follow-up Newborn, Section on Neurology, Section on Radiology. Routine
setting. J Clin Med. 2019;8(11):1790 neuroimaging of the preterm brain. Pediatrics. 2020;146(5):
60.Bosanquet M, Copeland L, Ware R, Boyd R. A systematic review of e2020029082
tests to predict cerebral palsy in young children. Dev Med Child 78. Helderman J, O’Shea TM, Dansereau L, et al. Association of
Neurol. 2013;55(5):418–426 abnormal findings on neonatal cranial ultrasound with
61. Romeo DM, Ricci D, Brogna C, Mercuri E. Use of the neurobehavior at neonatal intensive care unit discharge in infants
Hammersmith Infant Neurological Examination in infants with born before 30 weeks’ gestation. JAMA Netw Open. 2022;5(4):
cerebral palsy: a critical review of the literature. Dev Med Child e226561
Neurol. 2016;58(3):240–245 79. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and
62. Haataja L, Mercuri E, Regev R, et al. Optimality score for the evolution of subependymal and intraventricular hemorrhage: a study
neurologic examination of the infant at 12 and 18 months of age. J of infants with birth weights less than 1,500 gm. J Pediatr. 1978;
Pediatr. 1999;135(2 Pt 1):153–161 92(4):529–534
63. Romeo DMM, Cioni M, Palermo F, Cilauro S, Romeo MG. 80. Maitre NL, Marshall DD, Price WA, et al. Neurodevelopmental
Neurological assessment in infants discharged from a neonatal outcome of infants with unilateral or bilateral periventricular
intensive care unit. Eur J Paediatr Neurol. 2013;17(2):192–198 hemorrhagic infarction. Pediatrics. 2009;124(6):e1153–e1160
64. Haataja L, Mercuri E, Guzzetta A, et al. Neurologic examination in 81. Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE.
infants with hypoxic-ischemic encephalopathy at age 9 to 14 Neonatal MRI to predict neurodevelopmental outcomes in preterm
months: use of optimality scores and correlation with magnetic infants. N Engl J Med. 2006;355(7):685–694
resonance imaging findings. J Pediatr. 2001;138(3):332–337 82. Agut T, Alarcon A, Caba~
nas F, Bartocci M, Martinez-Biarge M,
65. Ljungblad UW, Paulsen H, Tangeraas T, Evensen KAI. Reference Horsch S; eurUS.brain Group. Preterm white matter injury:
material for Hammersmith Infant Neurologic Examination scores ultrasound diagnosis and classification. Pediatr Res. 2020;87:37–49
e10 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
83. Mirmiran M, Barnes PD, Keller K, et al. Neonatal brain magnetic 92. Te Velde A, Tantsis E, Novak I, et al. Age of diagnosis, fidelity and
resonance imaging before discharge is better than serial cranial acceptability of an early diagnosis clinic for cerebral palsy: a single
ultrasound in predicting cerebral palsy in very low birth weight site implementation study. Brain Sci. 2021;11(8):1074
preterm infants. Pediatrics. 2004;114(4):992–998 93. Maitre NL, Burton VJ, Duncan AF, et al. Network implementation
84. Ho T, Dukhovny D, Zupancic JAF, Goldmann DA, Horbar JD, of guideline for early detection decreases age at cerebral palsy
Pursley DM. Choosing wisely in newborn medicine: five diagnosis. Pediatrics. 2020;145(5):e20192126
opportunities to increase value. Pediatrics. 2015;136(2):e482–e489 94. Maitre NL, Damiano D, Byrne R. Implementation of early detection
85. George JM, Fiori S, Fripp J, et al. Validation of an MRI brain injury and intervention for cerebral palsy in high-risk infant follow-up
and growth scoring system in very preterm infants scanned at 29- to programs: U.S. and global considerations. Clin Perinatol.
35-week postmenstrual age. AJNR Am J Neuroradiol. 2017;38(7): 2023;50(1):269–279
1435–1442 95. Baird G, McConachie H, Scrutton D. Parents’ perceptions of
86. Parikh NA, Hershey A, Altaye M. Early detection of cerebral palsy disclosure of the diagnosis of cerebral palsy. Arch Dis Child.
using sensorimotor tract biomarkers in very preterm infants. Pediatr 2000;83(6):475–480
Neurol. 2019;98:53–60 96.Novak I, Morgan C, Fahey M, et al. State of the evidence traffic
87. Morgan C, Fahey M, Roy B, Novak I. Diagnosing cerebral palsy in lights 2019: systematic review of interventions for preventing and
full-term infants. J Paediatr Child Health. 2018;54(10):1159–1164 treating children with cerebral palsy. Curr Neurol Neurosci Rep.
88. Romeo DM, Guzzetta A, Scoto M, et al. Early neurologic assessment 2020;20(2):3
in preterm-infants: integration of traditional neurologic examination 97. Maitre NL, Byrne R, Duncan A, et al; CP EDI Consensus Group;
and observation of general movements. Eur J Paediatr Neurol. Canadian Neonatal Follow-up Network. “High-risk for cerebral
2008;12(3):183–189 palsy” designation: A clinical consensus statement. J Pediatr Rehabil
89. Maitre NL, Chorna O, Romeo DM, Guzzetta A. Implementation of Med. 2022;15(1):165–174
the Hammersmith Infant Neurological Examination in a high-risk 98. Byrne R, Duncan A, Pickar T, et al. Comparing parent and provider
infant follow-up program. Pediatr Neurol. 2016;65:31–38 priorities in discussions of early detection and intervention for
90.Byrne R, Noritz G, Maitre NL; NCH Early Developmental Group. infants with and at risk of cerebral palsy. Child Care Health Dev.
Implementation of early diagnosis and intervention guidelines for 2019;45(6):799–807
cerebral palsy in a high-risk infant follow-up clinic. Pediatr Neurol. 99.Maitre NL, Jeanvoine A, Yoder PJ, et al; BBOP group. Kinematic
2017;76:66–71 and somatosensory gains in infants with cerebral palsy after a multi-
91. King AR, Machipisa C, Finlayson F, Fahey MC, Novak I, Malhotra component upper-extremity intervention: A randomized controlled
A. Early detection of cerebral palsy in high-risk infants: Translation trial. Brain Topogr. 2020;33(6):751–766
of evidence into practice in an Australian hospital. J Paediatr Child 100.McNamara L, Morgan C, Novak I. Interventions for motor disorders
Health. 2021;57(2):246–250 in high-risk neonates. Clin Perinatol. 2023;50(1):121–155
Vol. 25 No. 1 JANUARY 2024 e11
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
ARTICLE
Nutritional Needs of the Infant with
Bronchopulmonary Dysplasia
Audrey N. Miller, MD,* Jennifer Curtiss, MS, RD, LD,† Matthew J. Kielt, MD*
*Comprehensive Center for Bronchopulmonary Dysplasia, Nationwide Children’s Hospital and Department of Pediatrics, The Ohio State University College
of Medicine, Columbus, OH
†
Department of Clinical Nutrition and Lactation, Nationwide Children’s Hospital, Columbus, OH
PRACTICE GAP
Infants with bronchopulmonary dysplasia (BPD) often experience postnatal
growth failure, which can affect neurodevelopment and short- and long-
term respiratory outcomes. High-quality evidence on how best to optimize
nutrition in the setting of BPD remains limited. However, recent reviews
and clinical guidance based on growth outcomes can help clinicians
improve growth in this vulnerable population.
OBJECTIVES After completing this article, readers should be able to:
1. Describe reasons for growth failure in infants with bronchopulmonary
dysplasia (BPD) and how they influence nutritional management.
2. Explain the enteral nutritional needs of infants with BPD throughout
disease progression.
3. Recognize the importance of growth monitoring and how it relates to
the management of BPD.
ABSTRACT
Growth failure is a common problem in infants with established
bronchopulmonary dysplasia (BPD). Suboptimal growth for infants with
BPD is associated with unfavorable respiratory and neurodevelopmental
outcomes; however, high-quality evidence to support best nutritional
AUTHOR DISCLOSURES Drs Miller and practices are limited for this vulnerable patient population. Consequently,
Kielt and Ms Curtiss have disclosed no there exists a wide variation in the provision of nutritional care and
financial relationships relevant to this
article. This commentary does not
monitoring of growth for infants with BPD. Other neonatal populations at
contain a discussion of an unapproved/ risk for growth failure, such as infants with congenital heart disease, have
investigative use of a commercial demonstrated improved growth outcomes with the creation and
product/device.
compliance of clinical protocols to guide nutritional management.
Developing clinical protocols to guide nutritional management for infants
ABBREVIATIONS with BPD may similarly improve long-term outcomes. Given the absence of
BPD bronchopulmonary dysplasia high-quality trials to guide nutritional practice in infants with BPD, the best
BPD-PH bronchopulmonary
available evidence of systematic reviews and clinical recommendations can
dysplasia–associated pulmonary
hypertension be applied to optimize growth and decrease variation in the care of these
MBD metabolic bone disease infants.
PMA postmenstrual age
e12 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
INTRODUCTION based nutritional care that improves long-term outcomes
Bronchopulmonary dysplasia (BPD) is the most common for infants with BPD.
sequelae of preterm birth, with a rising incidence reported
among infants born at or before 28 weeks’ gestation. (1) GROWTH AND NUTRITION IN BPD PREVENTION
Despite notable improvements in the respiratory care of Suboptimal growth is associated with adverse respiratory
infants born extremely preterm, rates of BPD are increas- outcomes for infants born preterm. (5)(6) Infants who ex-
ing, (2) likely secondary to the improving survival of perienced intrauterine growth restriction are at risk for de-
infants born extremely preterm, resulting in a growing layed fetal lung maturation, abnormal pulmonary vascular
population of infants and children with BPD in adult- angiogenesis, chronic fetal hypoxia, and increased airway
hood. BPD is commonly defined in infants born before resistance measured at a median postnatal age of 10
32 weeks’ gestation who require ongoing respiratory months. (17)(18) Infants born at very low birthweight have
support at 36 weeks’ postmenstrual age (PMA), with minimal energy reserves (body fat and glycogen stores)
the modality of respiratory support defining BPD severity. and limited stores of essential nutrients (antioxidant vita-
(3) Infants with grade 3 BPD require invasive mechanical mins A and E, iron, zinc, and long-chain polyunsaturated
ventilation at 36 weeks’ PMA, and are at the highest risk fatty acids) involved in lung injury defense and lung re-
for late death (before 18–26 months’ corrected age), seri- pair. (19)(20) Inadequate postnatal nutrition can lead to
ous respiratory morbidity, neurodevelopmental delay, and delayed somatic growth, delayed alveolar development,
suboptimal growth at follow-up. (3) and abnormal lung healing. (19)(20) Nutritional status has
To date, most nutritional research has centered on in- been shown to influence the development of BPD. Under-
fants born preterm who are at risk for BPD with less nutrition affects the ability of preterm infants to resist hy-
emphasis placed on those infants with established dis- peroxic damage, repair barotrauma-induced cell damage,
ease. (4) Suboptimal growth early in infancy is a well- fight infection, tolerate prolonged stress, and promote
established risk factor for the development of BPD. Preterm lung growth in experimental and clinical studies. (20)
infants who experience intrauterine growth restriction and Vitamin A deficiency can predispose an infant to the de-
are born small for gestational age are more likely to develop velopment of BPD as it is an important regulator of normal
BPD. (5)(6)(7) In addition, infants who develop BPD are at lung growth and is involved in maintaining the integrity of
risk for ongoing growth failure, abnormal growth patterns epithelial cells of the respiratory tract. (21)(22)(23)(24) Intra-
such as excess weight gain relative to linear growth, and muscular vitamin A supplementation has been shown to
delayed growth into adolescence and young adulthood. reduce the incidence of BPD. (21)(25) Vitamin D has anti-
(8)(9)(10) Identifying and addressing growth failure early is inflammatory properties and is important for lung growth
critical, as it has been shown to affect both short- and long- and repair. (26) Early supplementation of vitamin D (dur-
term outcomes. ing the first month of age) has been shown to reduce the
Owing to a gap in research centered on infants with es- incidence of BPD. (27) Inositol promotes endothelial cell
tablished BPD, the association of suboptimal growth with growth, acts as an antioxidant, and is involved in surfactant
adverse outcomes for infants with established BPD is not synthesis. A statistically significant reduction in mortality or
well understood. Evidence-based nutritional strategies for BPD has been reported in infants supplemented with inosi-
these infants are also not well-defined. Many factors com- tol. (28) Vitamin E, known to neutralize free radicals and
plicate achieving optimal nutrition and growth in infants reduce oxidative stress, has been studied in the pathophysi-
with BPD, including limited high-grade evidence to guide ology of BPD with vitamin E deficiency increasing BPD
clinical practice. This has led to differences among clini- risk. Vitamin E administration during the acute phase of re-
cians and centers in the nutritional delivery and monitoring spiratory distress syndrome has been shown to modify the
of infants with BPD. (11) However, even in the absence of ran- development of BPD. (29) Another study reported a correla-
domized controlled trials, there is growing interest in optimiz- tion between low vitamin E and selenium levels measured
ing multidisciplinary care of infants with BPD. An increasing in cord plasma and at days of age and the development of
body of literature exists, which includes nutritional reviews, BPD. (30) A review of vitamin E and its role in preventing
clinical practice guidelines, and published growth outcomes and treating BPD noted that while the findings from stud-
for this growing population. (4)(5)(12)(13)(14)(15)(16) Our ies of vitamin E supplementation are mixed, results may
objective in this review is to summarize the available nu- have been affected by vitamin E dose, route, and carrier prep-
tritional information to provide a framework for evidence- aration. (31) Several other nutritional factors such as selenium,
Vol. 25 No. 1 JANUARY 2024 e13
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
glutamine, human recombinant copper zinc superoxide dis- seen in the convalescing infant with BPD who is working
mutase, N-acetylcysteine, and zinc have been studied in rela- to establish full oral feedings. (4) Despite the need for in-
tion to BPD but have not been proven to prevent the disease. creased energy intake, infants with BPD may receive inad-
(32)(33) equate nutrition during periods of clinical instability or
BPD exacerbation. This can occur secondary to frequent
GROWTH AND NUTRITION-RELATED OUTCOMES discontinuation of feedings during periods of acute illness
IN BPD and chronic fluid restriction affecting nutrient delivery. In-
The association of suboptimal growth and adverse respira- terestingly, infants with BPD have been noted to have lower
tory outcomes continues past infancy. Children with BPD calorie and protein intake after the second week of age
who demonstrate catch-up growth, or above-average growth compared with preterm infants without BPD, suggesting
measured by weight gain, have been shown to have greater that nutritional deficiencies may indeed be a chronic prob-
longitudinal improvement in pulmonary function testing lem starting relatively soon after birth. (5)(46) Infants with
compared with children with BPD and average or below-av- chronic illness often have an altered metabolic state, mak-
erage weight gain. (34) Linear growth in particular is associ- ing total energy needs difficult to estimate, particularly
ated with improvement in lung function in children with when compared to healthy, age-matched controls. Conse-
BPD as noted on pulmonary function testing, even more so quently, more research is needed to determine the true en-
than an increase in weight. (35) Persistent lung hyperinfla- ergy needs in infants with established BPD. (47)(48)
tion at 4 to 8 years of age has been documented in children
with BPD who had poor nutritional status at 2 years of age, Medical Management
(36) suggesting that early nutritional interventions may po- Throughout the clinical course of infants with BPD, cer-
tentially modify severe disease phenotypes. tain aspects of medical management may further contrib-
Early and inadequate nutrition and lack of optimal ute to suboptimal growth (Fig 1). For example, systemic
growth also contribute to common morbidities observed corticosteroids are frequently used in both the prevention
in infants with BPD. Infants with BPD are at risk for neu- and management of BPD and are known to have a nega-
rodevelopmental impairment, (3) and although not specifi- tive impact on linear growth and cause lower weight gain
cally studied in BPD, there is a significant correlation velocity, independent of energy expenditure and energy
between poor postnatal growth and neurodevelopmental intake. (5)(49)(50)(51)(52) Systemic corticosteroids affect
impairment in infants born preterm. (37)(38)(39)(40) Un- growth at the cellular level by increasing protein catabo-
dernutrition can impair immune system function and pre- lism, reducing protein accretion, and altering lipid and bone
dispose infants to infections. (41) Respiratory infections metabolism. (48)(52)(53)(54) Diuretic therapy is commonly
and associated inflammation may exacerbate lung injury used in BPD to manage pulmonary edema, yet prolonged
in infants with BPD. (7) Preterm infants who experienced diuretic use can result in chronic electrolyte derangements
fetal growth restriction and/or are small for gestational that have a negative effect on growth and bone mineraliza-
age are at greater risk of developing BPD-associated pul- tion. (16)(55)(56) Infants with BPD are sensitive to rapid
monary hypertension (BPD-PH) and are at risk for pulmo- changes and frequent weaning of respiratory support that
nary hypertension crises and early mortality. (7) Infants may lead to increased levels of stress, work of breathing,
with BPD-PH who survive often show resolution of pul- and energy expenditure.
monary hypertension with lung growth. (7)
Comorbidities
GROWTH AND NUTRITION CHALLENGES IN Infection, a common comorbidity seen in infants with BPD,
INFANTS WITH ESTABLISHED BPD can increase energy expenditure, cause protein catabolism,
Energy Balance and reduce fatty acid oxidation. (16) Chronic inflammation
Infants with BPD require more total energy than age- as seen in infants with BPD can suppress insulinlike growth
matched controls without BPD, likely secondary to in- factors leading to growth suppression. (16) Some infants
creased work of breathing and increased metabolic de- with BPD develop significant edema that can lead to changes
mands. (42)(43)(44) This is especially true during the in enteral nutrition delivery and increased use of diuretic
acute phase of grade 3 BPD, characterized by high oxygen therapy. In addition, infants with BPD-PH are at risk for
needs, high levels of respiratory support, and respiratory suboptimal somatic growth compared with infants with BPD
instability. (4)(45) This increased energy need can also be and no pulmonary hypertension. (57)
e14 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
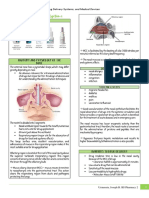
Frequent Needs vary
discontinuation of depending on:
enteral feedings
Chronic fluid • Disease severity
restriction • Chronic stress
Decreased Evolving Nutritional • Activity level
Energy Intake Needs • Oral feedings
Feeding Linear • Age
Growth
intolerance
Challenges
in BPD
Challenges with
oral feeding • Corticosteroids
• Diuretics
High Energy Medical • Inadequate
Expenditure Management respiratory support
• Stressful procedures
Sepsis • Chronic
Pneumonia Increased work of inflammation
breathing • Inaccurate length
measurements
Figure 1. Linear growth challenges seen in infants with bronchopulmonary dysplasia (BPD).
Evolving Nutritional Needs line–associated bloodstream infections and parenteral
The nutritional needs of the infant with BPD evolve over nutrition–associated cholestasis. (58)(59) Gastric feeding
time depending on clinical stability, level of respiratory is physiologic and ideal; 1 study found that transpyloric
support, age, and activity level. (4) Delivering targeted nu- feedings were associated with increased hypoxemic events
tritional interventions can therefore be quite challenging and a greater proportion of daily hypoxemia in infants with
to navigate, especially when infants experience BPD exac- BPD compared with gastric feedings. (60) Infants with grade
erbations with subsequent fluctuations in clinical stability. 3 BPD requiring tracheostomy will need long-term feeding
Four phases of BPD progression have been described with access, such as a gastrostomy tube, for ongoing nutritional
differing nutritional needs outlined in each phase. (45) support before they are discharged from the hospital. (61)
The first, or acute, phase is characterized by physiologic
instability, high oxygen needs, frequent respiratory work Fluid and Energy
of breathing and desaturations, and poor neuroregulation Fluid restriction in BPD remains a common practice, as
(Fig 2). The second, transitional, phase is characterized by higher fluid intake may contribute to pulmonary intersti-
slow weaning of supplemental oxygen, improved work of tial edema and lower lung compliance. (62) Although
breathing, brief periods of quiet alertness, and emerging there is no evidence to support this practice, (63) many
tolerance of low-level activities. The third, progrowth, centers continue to prescribe fluid restriction, especially
phase is seen with stable oxygen requirements, comfort- during the acute phase of BPD when the presence of ex-
able work of breathing, and the ability to wean respiratory cessive weight due to edema is commonly observed. (64) It
support. Changes in growth patterns may also be observed is important to ensure appropriate fluid balance to maintain
in the different phases. (Fig 2). As the infant progresses normal serum electrolyte concentrations as well as the de-
through these phases, fluid and energy intake, and protein livery of sufficient calories and protein.
needs gradually decrease. Recommendations for fluid, en- Energy needs vary based on the infant’s age, clinical se-
ergy, and protein intake (Fig 2) are based on clinical experi- verity, and activity level. Infants with BPD may require
ence in a 24-bed unit for infants with BPD, where significant more than 120 kcal/kg per day, especially in the acute
improvement in all growth parameters was documented us- phase of the disease. (4)(5)(12)(65) The higher energy
ing this method. (4)(51) needs compared with preterm infants without BPD are
often explained by higher estimated energy expenditures
ENTERAL NUTRITION IN INFANTS WITH secondary to increased work of breathing and oxygen re-
ESTABLISHED BPD quirements. (44)
Enteral feeding is preferred over parenteral nutrition and
should be prioritized even in the setting of acute illness, Protein
BPD exacerbations, and periods of sedation and paralysis. Adequate protein intake is essential to support lung develop-
(4) Parenteral nutrition places the infant at risk for central ment, lung repair, linear growth, and long-term improved
Vol. 25 No. 1 JANUARY 2024 e15
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
Figure 2. Characteristics, growth observations, and basic nutrition guidelines for infants with severe bronchopulmonary dysplasia based on disease
severity. Reproduced from Miller et al (4) originally published in the Journal of Perinatology.
pulmonary outcomes. Protein needs for infants with estab- formulas. If maternal milk is not available, preterm in-
lished BPD are higher than for preterm infants without fant formulas are recommended because of their higher
BPD. (66)(67) One study found that infants with BPD fed concentration of protein, minerals, and vitamins. (73) As
formula with added protein had improved linear growth, the infant nears term age, or shortly thereafter, postdi-
lean mass accretion, and greater bone mass. (68) scharge formulas can be used, as they contain higher
amounts of energy, protein, minerals, and vitamins com-
Feeding Selection pared with term infant formula. (73) If additional calories
Human milk is recommended as the ideal source of nutri- are needed above fortification, medium-chain triglycer-
tion for infants, exclusively until 6 months of age, and ides or liquid protein may be added, with close monitor-
then in combination with complementary food for up to ing of energy/protein ratio and osmolality. After 1 year
2 years of age or longer if desired. (69) Maternal milk of- of age, total energy intake may be supplemented with
fers many protective benefits and has been associated with
ready-to-feed pediatric formulas that are available in a
a lower incidence of developing BPD. (70)(71) A study in in-
30 kcal/ounce concentration. (74)
fants with established BPD demonstrated that those re-
ceiving long-term maternal milk had fewer systemic
ZINC AND ESTABLISHED BPD
corticosteroid courses, fewer emergency room visits, lower
There is growing interest in the role of zinc in infants
risk of readmissions, and reduced cough. (72) Preterm in-
fants with BPD require fortification of maternal milk (or with BPD. Zinc has several important functions, including
donor milk if being used) to provide essential nutrients in protein synthesis, enzyme function, tissue repair, cell
for catch-up growth and bone health. (12) division, neurotransmission, immune response, vision,
Nonetheless, owing to prolonged periods of critical ill- and growth. (75) Zinc deficiency is well-described in in-
ness during the initial birth hospitalization, provision of fants born preterm, small for gestational age, and with es-
maternal milk may be difficult for infants with established tablished BPD. (75)(76) This is likely secondary to low
BPD in the chronic phase of disease when maternal milk body stores due to reduced placental transfer, minimal
supplies may be low or no longer available. Although in- zinc intake after birth, and high zinc needs during rapid
fant formulas are readily available, the ideal alternative growth. Although the diagnosis of zinc deficiency can be
to human milk remains unknown as does the ideal age aided by the presence of dermatitis, diarrhea, infections,
at which to transition from preterm to term infant and neurologic disorders, these clinical manifestations are
e16 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
not often observed until there is a severe deficiency. (75) intubated infants; however, with routine staff education
Zinc biomarkers, such as serum alkaline phosphatase lev- and unit protocols in place, obtaining length measure-
els, have not been useful in determining zinc status. (77) ments using length boards should be standard of care. It
Knowing that preterm infants with BPD are at high risk is important to consistently obtain reliable length meas-
for zinc deficiency and insufficiency, zinc supplementa- urements using a pediatric length board. (82)(83) Length
tion should be considered for those demonstrating subop- measurements should be performed by 2 trained adults
timal growth in the setting of appropriate energy and and is often completed in combination by a nurse, dieti-
macronutrient intake. Infants receiving long-term diuretic tian, and parent. In our experience, parents enjoy helping
therapy should also be considered at risk for zinc defi- with this measurement and being involved in monitoring
ciency, as diuretics increase urinary zinc excretion. (78) their child’s growth.
Zinc supplementation in infants with BPD has been
shown to significantly improve both weight gain velocity Laboratory Monitoring
and linear growth. (79) Though zinc supplementation has Intermittent laboratory testing may help further assess nu-
not been rigorously studied in infants with established tritional status, especially for infants who are prescribed
BPD, observational data suggest that zinc supplementation fluid restriction, diuretics, and select vitamin and mineral
is likely benign. No documented side effects of zinc sup- supplements. Electrolyte monitoring is needed for infants
plementation in infants have been reported. Zinc toxicity receiving diuretic therapy, and these infants often require
is rare, and ingestion of up to 10 times the recommended sodium and potassium chloride supplementation. Addi-
daily intake produces no symptoms. (80) Chronic high tional laboratory testing such as blood urea nitrogen or
doses of zinc may be associated with copper deficiency, prealbumin (transthyretin) measurements can be used to
but this is rare at appropriate doses. (80) Nonetheless, re- monitor protein intake. (15)(84) Visceral protein levels,
search is needed to determine the most appropriate con- such as albumin, may reflect the severity of illness versus
centration, dose, and duration for zinc use in infants with nutritional status and can be confounded by postsurgical
established BPD. Our practice is to use compounded oral stress, liver disease, and kidney injury. (85) If there is con-
zinc acetate, at a strength of 10 mg/mL, often prescribed cern for iron deficiency, ferritin, hemoglobin, and hemato-
at 1 mg twice a day during hospitalization; we discontinue crit can be monitored as well.
zinc supplementation when diuretic therapy is stopped
and growth shows consistent improvement. Metabolic Bone Disease
Metabolic bone disease (MBD) is common in infants with
NUTRITIONAL AND GROWTH MONITORING BPD, with a reported prevalence of up to 60% at 36
weeks’ PMA. (86) Several factors place infants with BPD
Anthropometric Measurements
at risk for developing MBD, including preterm birth, post-
Routine discussion of overall growth status with the die-
natal nutritional management, and exposure to systemic
titian and multidisciplinary team is recommended. An-
corticosteroids and long-term loop diuretics. (87)(88) Con-
thropometric data, including weight, length, and head
sequences of MBD include short-term pain and discom-
circumference, should be monitored over time on age-
fort, as well as long-term growth implications such as
appropriate growth charts. Monitoring the weight-for-length
reduced bone mass and a higher risk for fracture and oste-
ratio is important to identify abnormal growth patterns. In-
oporosis. (89)(90)(91) Routine screening is recommended
creasing weight gain velocity out of proportion to linear
for infants at risk and includes serum concentrations
growth velocity may be indicative of edema or a significant
decrease in energy expenditure. (4) Mid-upper arm circum-
ference measurements may help differentiate edema from Table. Age-based Recommendations for Weight Gain
“true” weight gain, as the upper arm is less affected by Velocity and Linear Growth (101)
fluid status. (81) It is also important to consider that opti-
Weight gain Age ( mo) Recommendation ( g/d)
mal weight and linear growth ranges change as the patient 0–3 24–34
ages (Table). 3–6 13–21
Accurate length measurements are an essential compo- 6–12 8–11
Linear growth Age ( mo) Recommendation ( cm/wk)
nent of the nutrition assessment. Linear growth is a better 0–3 0.8–0.88
marker of nutrition status than weight gain alone. Obtain- 3–6 0.46–0.48
6–12 0.29–0.34
ing length measurements may be seen as challenging in
Vol. 25 No. 1 JANUARY 2024 e17
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
of alkaline phosphatase, calcium, and phosphorus. (92)
Management for MBD consists of enteral feeding adjust- Summary
ments and supplementation with calcium, phosphorus, and
• Growth failure in infants with BPD has important
vitamin D. (4)
short- and long-term implications that necessitate
a long-term multidisciplinary approach to
GROWTH AND RESPIRATORY SUPPORT
nutrition delivery and growth improvement. (95)
Routine growth assessments play a critical role in the re-
spiratory management of infants with BPD. For example,
• Implementation of nutritional clinical guidelines
growth trends help the team determine the timing of extu-
has led to improvement in growth outcomes in
bation or weaning of noninvasive positive pressure. At our other neonatal populations at risk for growth
institution, we like to see appropriate or improving growth failure, such as infants with congenital heart
assessments prior to a major respiratory support wean, disease. (48)(96)(97)(98)(99)(100) As we await
as linear growth is associated with successful respiratory high-quality evidence, clinicians are
weaning. (93) Some infants who have suboptimal growth encouraged to implement a standardized
on noninvasive positive pressure or nasal cannula second- approach to feeding infants with BPD using the
ary to persistent increased work of breathing and meta- available evidence and guidelines.
bolic demand will likely benefit from an increase in (4)(5)(12)(13)(14)(16)
respiratory support, even if this means a period of me-
chanical ventilation.
FUTURE DIRECTIONS American Board of Pediatrics
Many older studies have reported that infants with BPD Neonatal-Perinatal Content
require higher caloric intake than preterm infants without Specifications
BPD. (42)(43)(44)(94) However, these studies have limita-
• Know the pathogenesis, pathophysiology, and
tions such as small sample size, varied definitions of BPD,
pathologic features of bronchopulmonary
and imperfect energy measuring techniques such as the
dysplasia/chronic lung disease.
doubly labeled water technique and indirect calorimetry.
Indirect calorimetry testing may be useful for infants un- • Respiratory/H. Chronic lung disease,
dergoing invasive mechanical ventilation but can produce bronchopulmonary dysplasia/2. Know the
inaccurate results in the setting of air leaks, high fraction prenatal and postnatal risk factors for
of inspired oxygen needs above 0.6, and factors that affect bronchopulmonary dysplasia/chronic lung
steady state. (94) Further studies are needed to determine
disease and be aware of various preventive
the most accurate method of determining resting energy
strategies.
expenditure in this population. In addition, future studies • Recognize the clinical features of
should consider the patient’s age, activity level, respiratory bronchopulmonary dysplasia/chronic lung
status, BPD severity, and BPD phenotype when determin- disease.
ing energy needs. • Recognize the laboratory, radiographic, and
Although the evidence to suggest that infants with BPD other imaging features of bronchopulmonary
require more protein intake than preterm infants without dysplasia/chronic lung disease.
BPD is limited, (66)(67)(68) the amount and duration of
• Know the management of bronchopulmonary
the increased protein requirement remains unknown. Pro-
dysplasia/chronic lung disease.
spective randomized controlled trials may help determine
the optimal protein ranges for infants with established • Know the prognosis, long-term complications,
BPD at different age points. There are many studies on and permanent sequelae of bronchopulmonary
the role of nutrition in BPD prevention, however, future dysplasia.
studies are needed to determine if and how certain nu- • Know the caloric requirements for optimal
trients play a role in modifying the disease course of estab- postnatal growth of preterm and term infants,
lished BPD, such as vitamins A, D, and E as well as zinc, accounting for caloric expenditures needed for
and polyunsaturated fatty acids.
e18 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
10. Northway WH Jr, Moss RB, Carlisle KB, et al. Late pulmonary
physical activity and maintenance of body sequelae of bronchopulmonary dysplasia. N Engl J Med. 1990;
temperature. 323(26):1793–1799
11. Warren MG, Do B, Das A, et al; Eunice Kennedy Shriver National
• Know the clinical manifestations, diagnosis,
Institute of Child Health and Human Development Neonatal
management, and prevention of zinc, copper, Research Network. Gastrostomy tube feeding in extremely low
selenium, manganese, and chromium birthweight infants: frequency, associated comorbidities, and long-
deficiency. term outcomes. J Pediatr. 2019;214:41–46.e5
12. Bauer SE, Huff KA, Vanderpool CPB, Rose RS, Cristea AI. Growth
• Know the immunologic and anti-infective and nutrition in children with established bronchopulmonary
constituents in human milk and their dysplasia: a review of the literature. Nutr Clin Pract. 2022;37(2):
physiologic effects. 282–298
13. Bauer SE, Vanderpool CPB, Ren C, Cristea AI. Nutrition and growth
• Know that human milk needs to be fortified in
in infants with established bronchopulmonary dysplasia. Pediatr
order to meet the nutritional needs of preterm Pulmonol. 2021;56(11):3557–3562
infants. 14. Karatza AA, Gkentzi D, Varvarigou A. Nutrition of infants with
bronchopulmonary dysplasia before and after discharge from the
• Know the medical indications for the use of
neonatal intensive care unit. Nutrients. 2022;14(16):3311
non-standard infant formulas to meet the
15. Rocha G, Guimar~aes H, Pereira-da-Silva L. The role of nutrition in
needs of infants with special health problems. the prevention and management of bronchopulmonary dysplasia:
a literature review and clinical approach. Int J Environ Res Public
Health. 2021;18(12):6245
16. Curtiss J, Zhang H, Griffiths P, Shepherd EG, Lynch L.
Nutritional management of the infant with severe
References bronchopulmonary dysplasia. NeoReviews. 2015;16(12):e674–e679
doi: 10.1542/neo.16-12-e674
1. Stoll BJ, Hansen NI, Bell EF, et al; Eunice Kennedy Shriver National
Institute of Child Health and Human Development Neonatal 17. Bose C, Van Marter LJ, Laughon M, et al; Extremely Low Gestational
Research Network. Trends in care practices, morbidity, and mortality Age Newborn Study Investigators. Fetal growth restriction and
of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10): chronic lung disease among infants born before the 28th week of
1039–1051 gestation. Pediatrics. 2009;124(3):e450–e458
2. Bell EF, Hintz SR, Hansen NI, et al; Eunice Kennedy Shriver 18. Greenough A, Yuksel B, Cheeseman P. Effect of in utero growth
National Institute of Child Health and Human Development retardation on lung function at follow-up of prematurely born
Neonatal Research Network. Mortality, in-hospital morbidity, care infants. Eur Respir J. 2004;24(5):731–733
practices, and 2-year outcomes for extremely preterm infants in the 19. Ma L, Zhou P, Neu J, Lin HC. Potential nutrients for preventing or
US, 2013-2018. JAMA. 2022;327(3):248–263 treating bronchopulmonary dysplasia. Paediatr Respir Rev. 2017;22:
3. Jensen EA, Dysart K, Gantz MG, et al. The diagnosis of 83–88
bronchopulmonary dysplasia in very preterm infants. An 20. Frank L, Sosenko IR. Undernutrition as a major contributing factor
evidence-based approach. Am J Respir Crit Care Med. 2019; in the pathogenesis of bronchopulmonary dysplasia. Am Rev Respir
200(6):751–759 Dis. 1988;138(3):725–729
4. Miller AN, Curtiss J, Taylor SN, Backes CH, Kielt MJ. A review and 21. Darlow BA, Graham PJ, Rojas-Reyes MX. Vitamin A
guide to nutritional care of the infant with established supplementation to prevent mortality and short- and long-term
bronchopulmonary dysplasia. J Perinatol. 2023;43(3):402–410 morbidity in very low birth weight infants. Cochrane Database Syst
5. Poindexter BB, Martin CR. Impact of nutrition on Rev. 2016;2016(8):CD000501
bronchopulmonary dysplasia. Clin Perinatol. 2015;42(4): 22. Jensen EA, Laughon MM, DeMauro SB, et al. Contributions of the
797–806 NICHD neonatal research network to the diagnosis, prevention, and
6. Shima Y, Kumasaka S, Migita M. Perinatal risk factors for adverse treatment of bronchopulmonary dysplasia. Semin Perinatol.
long-term pulmonary outcome in premature infants: comparison of 2022;46(7):151638
different definitions of bronchopulmonary dysplasia/chronic lung 23. Rakshasbhuvankar AA, Pillow JJ, Simmer KN, Patole SK. Vitamin A
disease. Pediatr Int. 2013;55(5):578–581 supplementation in very-preterm or very-low-birth-weight infants to
7. Davidson LM, Berkelhamer SK. Bronchopulmonary dysplasia: prevent morbidity and mortality: a systematic review and meta-
chronic lung disease of infancy and long-term pulmonary outcomes. analysis of randomized trials. Am J Clin Nutr. 2021;114(6):
J Clin Med. 2017;6(1):4 2084–2096
8. Natarajan G, Johnson YR, Brozanski B, et al; Children’s Hospital 24. Tyson JE, Wright LL, Oh W, et al; National Institute of Child Health
Neonatal Consortium Executive Board and Study Group. Postnatal and Human Development Neonatal Research Network. Vitamin A
weight gain in preterm infants with severe bronchopulmonary supplementation for extremely-low-birth-weight infants. N Engl J
dysplasia. Am J Perinatol. 2014;31(3):223–230 Med. 1999;340(25):1962–1968
9. Clark RH, Thomas P, Peabody J. Extrauterine growth restriction 25. Massaro D, Massaro GD. Retinoids, alveolus formation, and alveolar
remains a serious problem in prematurely born neonates. Pediatrics. deficiency: clinical implications. Am J Respir Cell Mol Biol. 2003;
2003;111(5 Pt 1):986–990 28(3):271–274
Vol. 25 No. 1 JANUARY 2024 e19
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
26. Mandell E, Seedorf G, Gien J, Abman SH. Vitamin D treatment 43. Yeh TF, McClenan DA, Ajayi OA, Pildes RS. Metabolic rate and
improves survival and infant lung structure after intra-amniotic energy balance in infants with bronchopulmonary dysplasia.
endotoxin exposure in rats: potential role for the prevention of J Pediatr. 1989;114(3):448–451
bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 44. de Meer K, Westerterp KR, Houwen RH, Brouwers HA, Berger R,
2014;306(5):L420–L428 Okken A. Total energy expenditure in infants with
27. Ge H, Qiao Y, Ge J, et al. Effects of early vitamin D supplementation bronchopulmonary dysplasia is associated with respiratory status.
on the prevention of bronchopulmonary dysplasia in preterm infants. Eur J Pediatr. 1997;156(4):299–304
Pediatr Pulmonol. 2022;57(4):1015–1021 45. Logan JW, Lynch SK, Curtiss J, Shepherd EG. Clinical phenotypes
28. Howlett A, Ohlsson A, Plakkal N. Inositol for respiratory distress and management concepts for severe, established
syndrome in preterm infants at risk for or having respiratory bronchopulmonary dysplasia. Paediatr Respir Rev. 2019;31:58–63
distress syndrome. Cochrane Database Syst Rev. 2012;(3):CD000366; 46. Jiang H, Lv Y, Hou W, et al. Association between neonatal
Update in. Cochrane Database Syst Rev. 2015; (2):CD000366 malnutrition and bronchopulmonary dysplasia in very low-birth-
29. Ehrenkranz RA, Bonta BW, Ablow RC, Warshaw JB. Amelioration weight infants: a propensity score-matched analysis. Nutr Clin Pract.
of bronchopulmonary dysplasia after vitamin E administration: a 2022;37(6):1429–1437
preliminary report. N Engl J Med. 1978;299(11):564–569 47. Mehta NM. Energy expenditure: how much does it matter in infant
30. Falciglia HS, Johnson JR, Sullivan J, et al. Role of antioxidant and pediatric chronic disorders? Pediatr Res. 2015;77(1-2):168–172
nutrients and lipid peroxidation in premature infants with 48. Mehta NM, Skillman HE, Irving SY, et al. Guidelines for the
respiratory distress syndrome and bronchopulmonary dysplasia. Am provision and assessment of nutrition support therapy in the
J Perinatol. 2003;20(2):97–107 pediatric critically ill patient: Society of Critical Care Medicine and
31. Stone CA Jr, McEvoy CT, Aschner JL, et al. Update on vitamin E American Society for Parenteral and Enteral Nutrition. Pediatr Crit
and its potential role in preventing or treating bronchopulmonary Care Med. 2017;18(7):675–715
dysplasia. Neonatology. 2018;113(4):366–378 49. Leitch CA, Ahlrichs J, Karn C, Denne SC. Energy expenditure and
energy intake during dexamethasone therapy for chronic lung
32. Beam KS, Aliaga S, Ahlfeld SK, Cohen-Wolkowiez M, Smith PB,
disease. Pediatr Res. 1999;46(1):109–113
Laughon MM. A systematic review of randomized controlled trials
for the prevention of bronchopulmonary dysplasia in infants. 50. Lewis T, Truog W, Nelin L, Napolitano N, McKinney RL.
J Perinatol. 2014;34(9):705–710 Pharmacoepidemiology of drug exposure in intubated and non-
intubated preterm infants with severe bronchopulmonary dysplasia.
33. Biniwale MA, Ehrenkranz RA. The role of nutrition in the
Front Pharmacol. 2021;12:695270
prevention and management of bronchopulmonary dysplasia. Semin
Perinatol. 2006;30(4):200–208 51. Kielt MJ, Logan JW, Backes CH, Reber KM, Nelin LD, Shepherd
EG. In-hospital outcomes of late referrals for established
34. Filbrun AG, Popova AP, Linn MJ, McIntosh NA, Hershenson MB.
bronchopulmonary dysplasia. J Perinatol. 2021;41(8):1972–1982
Longitudinal measures of lung function in infants with
bronchopulmonary dysplasia. Pediatr Pulmonol. 2011;46(4):369–375 52. Van Goudoever JB, Wattimena JD, Carnielli VP, Sulkers EJ,
Degenhart HJ, Sauer PJ. Effect of dexamethasone on protein
35. Sanchez-Solis M, Perez-Fernandez V, Bosch-Gimenez V, Quesada
metabolism in infants with bronchopulmonary dysplasia. J Pediatr.
JJ, Garcia-Marcos L. Lung function gain in preterm infants with and
1994;124(1):112–118
without bronchopulmonary dysplasia. Pediatr Pulmonol. 2016;51(9):
936–942 53. Appel B, Fried SK. Effects of insulin and dexamethasone on
lipoprotein lipase in human adipose tissue. Am J Physiol. 1992;262(5
36. Bott L, B!eghin L, Devos P, Pierrat V, Matran R, Gottrand F.
Pt 1):E695–E699
Nutritional status at 2 years in former infants with
54. Hauner H, Schmid P, Pfeiffer EF. Glucocorticoids and insulin
bronchopulmonary dysplasia influences nutrition and pulmonary
promote the differentiation of human adipocyte precursor cells into
outcomes during childhood. Pediatr Res. 2006;60(3):340–344
fat cells. J Clin Endocrinol Metab. 1987;64(4):832–835
37. Skinner AM, Narchi H. Preterm nutrition and neurodevelopmental
55. Roberts K, Stepanovich G, Bhatt-Mehta V, Donn SM. New
outcomes. World J Methodol. 2021;11(6):278–293
pharmacologic approaches to bronchopulmonary dysplasia. J Exp
38. Meyers JM, Tan S, Bell EF, et al; Eunice Kennedy Shriver National Pharmacol. 2021;13:377–396
Institute of Child Health and Human Development Neonatal
56. Wassner SJ. Altered growth and protein turnover in rats fed sodium-
Research Network. Neurodevelopmental outcomes among extremely
deficient diets. Pediatr Res. 1989;26(6):608–613
premature infants with linear growth restriction. J Perinatol.
2019;39(2):193–202 57. Hansmann G, Sallmon H, Roehr CC, Kourembanas S, Austin ED,
Koestenberger M; European Pediatric Pulmonary Vascular Disease
39. Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole
Network (EPPVDN). Pulmonary hypertension in bronchopulmonary
WK. Growth in the neonatal intensive care unit influences
dysplasia. Pediatr Res. 2021;89(3):446–455
neurodevelopmental and growth outcomes of extremely low birth
weight infants. Pediatrics. 2006;117(4):1253–1261 58. Pulido AV, Kellogg K, Hunt K, Davis C. Development of a clinical
practice guideline for weaning and discontinuing parenteral
40. Pfister KM, Ramel SE. Linear growth and neurodevelopmental
nutrition in hospitalized children as part of a central line-associated
outcomes. Clin Perinatol. 2014;41(2):309–321 bloodstream infection-focused quality improvement initiative. JPEN
41. Katona P, Katona-Apte J. The interaction between nutrition and J Parenter Enteral Nutr. 2021;45(8):1653–1662
infection. Clin Infect Dis. 2008;46(10):1582–1588 59. Greenberg J, Naik M, Chapman J, Davidson A, Imseis E.
42. Kurzner SI, Garg M, Bautista DB, et al. Growth failure in infants Comparison of two lipid emulsions on the incidence of parenteral
with bronchopulmonary dysplasia: nutrition and elevated resting nutrition associated cholestasis in neonates. J Pediatr Pharmacol
metabolic expenditure. Pediatrics. 1988;81(3):379–384 Ther. 2023;28(2):129–135
e20 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
60.Jensen EA, Zhang H, Feng R, et al. Individualising care in severe 79. Shaikhkhalil AK, Curtiss J, Puthoff TD, Valentine CJ. Enteral zinc
bronchopulmonary dysplasia: a series of N-of-1 trials comparing supplementation and growth in extremely-low-birth-weight infants
transpyloric and gastric feeding. Arch Dis Child Fetal Neonatal Ed. with chronic lung disease. J Pediatr Gastroenterol Nutr. 2014;58(2):
2020;105(4):399–404 183–187
61. Murthy K, Porta NFM, Lagatta JM, et al. Inter-center variation in 80. Abrams SA. Zinc deficiency and supplementation in children.
death or tracheostomy placement in infants with severe Available at: https://www.uptodate.com/contents/zinc-deficiency-
bronchopulmonary dysplasia. J Perinatol. 2017;37(6):723–727 and-supplementation-in-children?search=Abrams%20SA.%20Zinc%
20deficiency%20and%20supplementation%20in%20children&
62. Bozzetti V, De Angelis C, Tagliabue PE. Nutritional approach to
source=search_result&selectedTitle=1!150&usage_type=default&
preterm infants on noninvasive ventilation: An update. Nutrition.
display_rank=1. Accessed October 12, 2023
2017;37:14–17
81. Pereira-da-Silva L, Virella D, Fusch C. Nutritional assessment in
63. Barrington KJ, Fortin-Pellerin E, Pennaforte T. Fluid restriction for
preterm infants: a practical approach in the NICU. Nutrients.
treatment of preterm infants with chronic lung disease. Cochrane Database
2019;11(9):1999
Syst Rev. 2017;2(2):CD005389 10.1002/14651858.CD005389.pub2
82. Arigliani M, Spinelli AM, Liguoro I, Cogo P. Nutrition and lung
64. Bamat NA, Zhang H, McKenna KJ, Morris H, Stoller JZ, Gibbs K.
growth. Nutrients. 2018;10(7):919
The clinical evaluation of severe bronchopulmonary dysplasia.
NeoReviews. 2020;21(7):e442–e453 83. Fenton TR, Anderson D, Groh-Wargo S, Hoyos A, Ehrenkranz RA,
Senterre T. An attempt to standardize the calculation of growth
65. Denne SC. Energy expenditure in infants with pulmonary
velocity of preterm infants-evaluation of practical bedside methods.
insufficiency: is there evidence for increased energy needs? J Nutr.
J Pediatr. 2018;196:77–83
2001;131(3):935S–937S
opez-Herce J, Menc!ıa S, Urbano J, Solana MJ, Garc!ıa A.
84. Botr!an M, L!
66.Groothuis JR, Makari D. Definition and outpatient management of
Enteral nutrition in the critically ill child: comparison of standard
the very low-birth-weight infant with bronchopulmonary dysplasia.
and protein-enriched diets. J Pediatr. 2011;159(1):27–32.e1
Adv Ther. 2012;29(4):297–311
85. Ong C, Han WM, Wong JJ, Lee JH. Nutrition biomarkers and
67. Giann"ı ML, Roggero P, Colnaghi MR, et al. The role of nutrition in
clinical outcomes in critically ill children: A critical appraisal of the
promoting growth in pre-term infants with bronchopulmonary
literature. Clin Nutr. 2014;33(2):191–197
dysplasia: a prospective non-randomised interventional cohort study.
BMC Pediatr. 2014;14:235 86. Gaio P, Verlato G, Daverio M, et al. Incidence of metabolic bone
disease in preterm infants of birth weight <1250 g and in those
68. Brunton JA, Saigal S, Atkinson SA. Growth and body composition
suffering from bronchopulmonary dysplasia. Clin Nutr ESPEN.
in infants with bronchopulmonary dysplasia up to 3 months 2018;23:234–239
corrected age: a randomized trial of a high-energy nutrient-enriched
87. Chen W, Zhang Z, Dai S, Xu L. Risk factors for metabolic
formula fed after hospital discharge. J Pediatr. 1998;133(3):340–345
bone disease among preterm infants less than 32 weeks
69.Meek JY, Noble L; Section on Breastfeeding. Policy statement: gestation with Bronchopulmonary dysplasia. BMC Pediatr.
breastfeeding and the use of human milk. Pediatrics. 2022;150(1): 2021;21(1):235
e2022057988
88. Jensen EA, White AM, Liu P, et al. Determinants of severe
70. Huang J, Zheng Z, Zhao XY, et al. Short-term effects of fresh mother’s metabolic bone disease in very low-birth-weight infants with severe
own milk in very preterm infants. Matern Child Nutr. 2023;19(1):e13430 bronchopulmonary dysplasia admitted to a tertiary referral center.
71. Xu Y, Yu Z, Li Q, et al. Dose-dependent effect of human milk on Am J Perinatol. 2016;33(1):107–113
Bronchopulmonary dysplasia in very low birth weight infants. BMC 89. Chinoy A, Mughal MZ, Padidela R. Metabolic bone disease of
Pediatr. 2020;20(1):522 prematurity: causes, recognition, prevention, treatment and long-
72. Kim LY, McGrath-Morrow SA, Collaco JM. Impact of breast milk on term consequences. Arch Dis Child Fetal Neonatal Ed. 2019;
respiratory outcomes in infants with bronchopulmonary dysplasia. 104(5):F560–F566
Pediatr Pulmonol. 2019;54(3):313–318 90.Wood CL, Wood AM, Harker C, Embleton ND. Bone mineral
73. Morgan JA, Young L, McCormick FM, McGuire W. Promoting density and osteoporosis after preterm birth: the role of early life
growth for preterm infants following hospital discharge. Arch Dis factors and nutrition. Int J Endocrinol. 2013;2013:902513
Child Fetal Neonatal Ed. 2012;97(4):F295–F298 91. Pravia CI, Benny M. Long-term consequences of prematurity. Cleve
74. Anderson C, Hillman NH. bronchopulmonary dysplasia: when the Clin J Med. 2020;87(12):759–767
very preterm baby comes home. Mo Med. 2019;116(2):117–122 92. Abrams SA; Committee on Nutrition. Calcium and vitamin d
75. Terrin G, Berni Canani R, Di Chiara M, et al. Zinc in early life: a requirements of enterally fed preterm infants. Pediatrics.
key element in the fetus and preterm neonate. Nutrients. 2013;131(5):e1676–e1683
2015;7(12):10427–10446 93. Miller AN, Moise AA, Cottrell L, Loomis K, Polak M, Gest A. Linear
76. V!azquez-Gomis R, Bosch-Gimenez V, Juste-Ruiz M, V!azquez- growth is associated with successful respiratory support weaning in
Gomis C, Izquierdo-Fos I, Pastor-Rosado J. Zinc concentration in infants with bronchopulmonary dysplasia. J Perinatol.
preterm newborns at term age, a prospective observational study. 2022;42(4):544–545
BMJ Paediatr Open. 2019;3(1):e000527 94. Bott L, B!eghin L, Marichez C, Gottrand F. Comparison of resting
77. Lowe NM, Fekete K, Decsi T. Methods of assessment of zinc status energy expenditure in bronchopulmonary dysplasia to predicted
in humans: a systematic review. Am J Clin Nutr. 2009;89(6): equation. Eur J Clin Nutr. 2006;60(11):1323–1329
2040S–2051S 95. Abman SH, Collaco JM, Shepherd EG, et al; Bronchopulmonary
78. Cohen N, Golik A. Zinc balance and medications commonly used in Dysplasia Collaborative. Interdisciplinary care of children with severe
the management of heart failure. Heart Fail Rev. 2006;11(1):19–24 bronchopulmonary dysplasia. J Pediatr. 2017;181:12–28.e1
Vol. 25 No. 1 JANUARY 2024 e21
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
96.Marino LV, Johnson MJ, Hall NJ, et al; British Dietetic Association 99.Jaksic T, Hull MA, Modi BP, Ching YA, George D, Compher C;
Paediatric Cardiology Interest Group. The development of a American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.)
consensus-based nutritional pathway for infants with CHD before Board of Directors. A.S.P.E.N. Clinical guidelines: nutrition support
surgery using a modified Delphi process. Cardiol Young. of neonates supported with extracorporeal membrane oxygenation.
2018;28(7):938–948 JPEN J Parenter Enteral Nutr. 2010;34(3):247–253
97. Mills KI, Kim JH, Fogg K, et al. Nutritional considerations for the 100. Tume LN, Valla FV, Joosten K, et al. Nutritional support for children
neonate with congenital heart disease. Pediatrics. 2022;150(suppl during critical illness: European Society of Pediatric and Neonatal
2):e2022056415G Intensive Care (ESPNIC) metabolism, endocrine and nutrition
98. Slicker J, Hehir DA, Horsley M, et al; Feeding Work Group of the section position statement and clinical recommendations. Intensive
National Pediatric Cardiology Quality Improvement Collaborative. Care Med. 2020;46(3):411–425
Nutrition algorithms for infants with hypoplastic left heart 101. Institute of Medicine. Dietary Reference Intakes: The Essential Guide to
syndrome; birth through the first interstage period. Congenit Heart Nutrient Requirements. Washington, DC: National Academies Press;
Dis. 2013;8(2):89–102 2006
e22 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
NEOREVIEWS QUIZ
NEO
QUIZ
1. Bronchopulmonary dysplasia (BPD) is a common complication of prematurity.
Suboptimal postnatal nutrition has been shown to be a risk factor for the
development of BPD. Very-low-birthweight infants, in particular, have minimal
energy reserves and limited stores of essential nutrients. All of the following
essential nutrients have been shown to be involved in lung defense and lung
repair EXCEPT:
A. Glycogen.
B. Iron.
C. Long-chain polyunsaturated fatty acids.
D. Vitamin A.
E. Zinc.
REQUIREMENTS: Learners can
2. Systemic corticosteroids are commonly used for the medical management of
take NeoReviews quizzes and
infants with BPD and have been shown to result in lower linear growth as claim credit online only at:
well as decreased weight gain velocity. Which of the following statements https://publications.aap.org/
describing the impact of systemic corticosteroids on growth is INCORRECT? neoreviews.
A. Altered lipid metabolism. To successfully complete 2024
B. Altered bone metabolism. NeoReviews articles for AMA PRA
C. Decreased protein accretion. Category 1 Credit™, learners
D. Increased fatty acid oxidation. must demonstrate a minimum
performance level of 60% or
E. Increased protein catabolism. higher on this assessment. If
3. Infections and chronic inflammation are comorbidities of BPD that have you score less than 60% on the
assessment, you will be given
been shown to increase energy expenditure and contribute to suboptimal
additional opportunities to
growth in these patients. Which of the following statements describes an answer questions until an
effect of chronic inflammation that contributes to poor growth in these overall 60% or greater score is
patients? achieved.
A. Increased zinc levels. This journal-based CME activity
B. Decreased insulinlike growth factor. is available through Dec. 31,
C. Decreased sodium reabsorption. 2026, however, credit will be
D. Decreased lipid oxidation. recorded in the year in which
the learner completes the quiz.
E. Reduced fatty acid oxidation.
4. The nutritional needs of infants with BPD vary over time, depending on the
age of the infant and the stage of BPD. In the acute phase of BPD,
characterized by physiologic instability, poor linear growth is observed
alongside variable weight trends. In the transitional phase, weight trend and
linear growth improve while consistent linear growth and improved weight- 2024 NeoReviews is approved
for-length trend are seen once the infant reaches the progrowth phase. for a total of 30 Maintenance of
Certification (MOC) Part 2
Which of the following statements describes a recommended nutritional
credits by the American Board
goal for an infant in the acute phase of BPD? of Pediatrics (ABP) through the
A. Arachidonic acid intake of 5 mg/kg per day. AAP MOC Portfolio Program.
NeoReviews subscribers can
B. Calcium intake of 80 mg/kg per day. claim up to 30 ABP MOC Part 2
C. Energy requirement of 150 mL/kg per day. points upon passing 30 quizzes
D. Protein intake of 3 g/kg per day. (and claiming full credit for
E. Total fluid goal of 110 mL/kg per day. each quiz) per year. Subscribers
can start claiming MOC credits
as early as October 2024. To
learn how to claim MOC points,
go to: https://publications.aap.
org/journals/pages/moc-credit.
Vol. 25 No. 1 JANUARY 2024 e23
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
5. Metabolic bone disease (MBD) is a common complication of prematurity.
Infants with BPD are at particularly high risk because of suboptimal postnatal
nutrition and exposure to systemic corticosteroids and diuretics. What is the
percentage of infants with BPD affected by MBD at 36 weeks’ postmenstrual
age?
A. 40%.
B. 50%.
C. 60%.
D. 70%.
E. 80%.
e24 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
ARTICLE
Optimizing Nutrition in Neonates with
Kidney Dysfunction
Saudamini Nesargi, DNB,* Heidi Steflik, MD, MSCR,† Nivedita Kamath, MD, DNB, DM,‡ David Selewski, MD, MS,§
Katja M. Gist, DO, MSCS,¶ Shina Menon, MDk
*Department of Neonatology, St. Johns Medical College Hospital, Bangalore, India
†
Division of Neonatology, Department of Pediatrics, Medical University of South Carolina, Charleston, SC
‡
Department of Pediatric Nephrology, St. Johns Medical College Hospital, Bangalore, India
§
Division of Nephrology, Department of Pediatrics, Medical University of South Carolina, Charleston, SC
¶
Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati
College of Medicine, Cincinnati, OH
k AUTHOR DISCLOSURES Dr Steflik works
Department of Pediatrics, Seattle Children’s Hospital, University of Washington School of Medicine,
under a grant from Baxter, has received
Seattle, WA
honoraria for speaking and reviewing at the
Medical University of South Carolina and
MedLink Neurology, respectively, and is an
unpaid board member for the Southern
Society of Pediatric Research. Dr Gist works
PRACTICE GAP under a grant from The Gerber Foundation
and has received consulting fees from
Neonates with kidney disease have complex nutritional requirements Bioporta Diagnostics and Portero Medical.
based on their underlying kidney dysfunction. New clinical guidelines are Dr Menon works under a grant from The
Gerber Foundation and has received
available, but they focus on children of all ages and are not exclusive to consulting fees from Medtronic and Nuwellis.
neonates. Therefore, significant gaps remain, particularly with respect to Dr Selewski works under a grant from and
acute kidney injury and renal replacement therapy in the neonatal has served on an advisory board for
Pharmacosmos. Drs Nesargi and Kamath
population.
disclosed no financial relationships relevant
to this article. This commentary does not
contain a discussion of an unapproved/
OBJECTIVES After completing this article, readers should be able to: investigative use of a commercial product/
device. Drs Nesargi and Steflik contributed
equally to this work.
1. Summarize the pathophysiology of compromised nutrition in neonates
with kidney disease and discuss the key nutritional aspects of acute
ABBREVIATIONS
kidney injury and chronic kidney disease.
AKI acute kidney injury
2. Assess the nutritional needs of neonates with chronic kidney disease ARA arachidonic acid
and apply the Pediatric Renal Nutrition Taskforce clinical practice BMI body mass index
BSA body surface area
recommendations. CKD chronic kidney disease
CRRT continuous renal replacement
3. Recognize the nutritional implications of the use of continuous renal
therapy
replacement therapy for acute kidney injury in neonates. DHA docosahexaenoic acid
EN enteral nutrition
ESPGHAN European Society for Pediatric
ABSTRACT Gastroenterology, Hepatology,
and Nutrition
The nutritional management of neonates with kidney disease is GIR glucose infusion rate
complex. There may be significant differences in nutritional needs based iHD intermittent hemodialysis
IV intravenous
on the duration and cause of kidney dysfunction, including acute kidney KDOQI Kidney Disease Outcomes
injury (AKI) and chronic kidney disease (CKD). Furthermore, the Quality Initiative
treatment modality, including acute (continuous renal replacement PD peritoneal dialysis
PN parenteral nutrition
therapy and peritoneal dialysis [PD]) and chronic (intermittent PRNT Pediatric Renal Nutrition
hemodialysis and PD) approaches may differentially affect nutritional Taskforce
losses and dietary needs. In this review, we discuss the pathophysiology REE resting energy expenditure
RRT renal replacement therapy
of compromised nutrition in neonates with AKI and CKD. We also
SDI suggested dietary intake
Vol. 25 No. 1 JANUARY 2024 e25
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
summarize the existing data and consensus recommendations on the provision of nutrition to neonates with AKI and
CKD. We highlight the paucity of data on micronutrient losses and the need for future prospective studies to enhance
nutritional supplementation to hopefully improve outcomes in these patients.
INTRODUCTION Preterm Neonates
Nutritional requirements during the neonatal period are In preterm infants, postnatal growth should approximate
of particular importance as growth rates are higher during the growth of a fetus of the same gestational age in utero.
infancy. In critically ill neonates, this is often compounded The recommended energy and carbohydrate prescriptions
by multiple complications related to their underlying path- vary depending on the route of feeding and individual pa-
ophysiology; therefore, nutrition should be individualized tient parameters including weight, clinical status, and es-
to optimize the neonates’ nutritional status and growth. tablished growth pattern, and thus must be individualized
(Table 1). (1) In all cases, energy intake in preterm infants
Neonates with kidney dysfunction represent one such
must equal the sum of energy expenditure. This includes
group with unique nutritional needs relative to other criti-
energy excreted through losses in stool and urine; energy
cally ill neonatal populations.
expended in basal metabolism, thermoregulation, activity,
The nutritional considerations in neonates with kidney
and new tissue synthesis; and energy stored as fat, pro-
dysfunction encompass a broad spectrum related not only
tein, and glycogen. (1) Significant variations exist in energy
to the acuity or chronicity of kidney dysfunction but also
and carbohydrate requirements even in preterm infants.
to the unique aspects of renal replacement therapy (RRT).
The 2022 European Society for Pediatric Gastroenterology,
Significant differences in nutritional needs in kidney dis-
Hepatology, and Nutrition (ESPGHAN) position paper on
ease based on the duration and cause of kidney dysfunc-
enteral nutrition (EN) in preterm infants recommends a
tion including acute kidney injury (AKI) and chronic
total energy intake of 115 to 140 kcal/kg per day for enter-
kidney disease (CKD) exist. As the epidemiology and im-
ally fed preterm infants who are healthy and growing,
pact of neonatal AKI have become clearer, there has been
though more than 140 kcal/kg per day may be needed in
a push to optimize the care of these patients, including
some cases. (2) Parenterally supported infants require
nutritional support.
fewer kilocalories (as a result of reduced energy costs for nu-
In this review, we will briefly examine nutrition in
trient absorption and reduced fecal energy losses), whereas
healthy neonatal populations, describe the pathophysiology
enterally fed neonates require more kilocalories because of
and nutritional considerations in neonates with AKI and increased diet-induced thermogenesis and increased fecal
CKD, and highlight areas for future research. energy losses. (2)
NUTRITIONAL NEEDS OF HEALTHY NEONATES Sources of Energy
The provision of nutrition to neonates depends on numer- Glucose is the body’s major energy source and is particu-
ous factors including gestational age, degree of illness and larly important for the brain and heart. However, lactose
comorbidities, postnatal age, and method of feeding. Herein, is the primary carbohydrate found in human milk. Lactose
we provide a summary that is separated into preterm and is incompletely digested in preterm infants, leading to
term neonates. concerns of increased risk for necrotizing enterocolitis
Table 1. Recommended Nutritional Intakes Based on Estimated Nutritional Needs in Healthy Term and Preterm
Infants
Nutritional Components Term Infants Preterm Infants
Energy 75–85 kcal/kg per day (maximum: 100 kcal/kg per day) 90–130 kcal/kg per day
Enteral: 10–14 g/kg/day
Parenteral (Glucose Infusion Rate): 2.5–10 mg/kg/min
Carbohydrates 2.5–10 mg/kg per minute 4–10 mg/kg per minute
Enteral: 11–15 g/kg/day
Parenteral (Glucose Infusion Rate): 4–10 mg/kg/min
Protein and amino acids 1.5–2.5 g/kg per day 2.5–4.5 g/kg per day
Lipids 5–6 g/kg per day 4.1–7.4 g/100 kcal
e26 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
secondary to carbohydrate malabsorption in this popula- to 7.4 g of fat per 100 kcal, depending on the fat content
tion. However, the substitution of lactose with more read- of the feedings. There are significant intra- and interindi-
ily digestible glucose has not been shown to improve vidual variations in human milk lipid content. In enterally
feeding tolerance or weight gain in preterm infants. (2) fed preterm neonates, ESPGHAN recommends a total fat
Full enteral carbohydrates in preterm neonates should to- intake of 4.8 to 8.1 g/kg per day but notes higher intake
tal 11 to 15 g/kg per day. (2) In parenterally fed preterm may be safe. (2) Long-chain polyunsaturated fatty acids, es-
neonates, glucose supplementation should provide appropri- pecially arachidonic acid (ARA) and docosahexaenoic acid
ate calories for basal glucose metabolism while maintaining (DHA) are critical to supplement in preterm neonates, as
a normal plasma glucose concentration (ie, 54–108 mg/dL). active placental transfer of these important fatty acids oc-
In term newborns, this typically requires a glucose infusion curs during the third trimester of pregnancy. These fatty
rate (GIR) of 6 to 8 mg/kg per minute, but in very-low-birth- acids, when supplied in appropriate amounts to preterm
weight preterm infants who are at risk for significant hyper- infants (DHA intake of 0.5%–1% of total fatty acids,
glycemia, lower initial rates of 3 to 6 mg/kg per minute may !30–65 mg/kg per day; dietary ARA/DHA ratio of 0.5–2),
be more appropriate. (3) GIR can safely be advanced daily by are associated with improved short-term neurologic out-
1 to 2 mg/kg per minute to a goal of 7 to 10 mg/kg per mi- comes. (2)(6) In preterm infants requiring PN, lipids are
nute if serum glucose levels remain within normal limits. typically initiated at 1 to 2 g/kg per day of intravenous (IV)
Notably, higher GIR (ie, 9–12 mg/kg per minute or higher) lipid emulsion and can be advanced to 2 to 3 g/kg per day
may be needed in very preterm or growth-restricted neonates as tolerated.
with hyperinsulinism-like disorders.
Proteins are vital for adequate growth as they are the Term Neonates
key structural component of all human cells and the main Nutrition prescriptions in term neonates are more straight-
driver of lean body mass growth. In preterm newborns, forward than in preterm neonates. EN should be provided
protein supplementation should begin immediately after with human milk, and formula should be used if essential.
birth at 1.5 to 2.5 g/kg per day. After 1 to 2 days, the pro- (7) PN may be needed in term infants such as those with
tein prescription can be advanced to 3.5 g/kg per day. (4) preexisting congenital anomalies of the gastrointestinal
In extremely preterm newborns, a combination of EN and tract or in those with or at risk for intestinal malperfusion
parenteral nutrition (PN) is provided, with slow uptitration (ie, some neonates with critical congenital heart disease).
of EN accompanied by downtitration of PN over the first The daily recommended energy requirement for term neo-
weeks of age. In transitioning from primarily PN to EN, nates varies from 75 to 120 kcal/kg per day and 105 to 130
first-pass amino acid metabolism must be considered, and kcal/kg per day depending on the guidelines (Table 1).
protein content in PN should not be downtitrated before (8)(9) There are several factors that may alter this require-
enteral intake exceeds 75 mL/kg per day and not discontin- ment, including the use of PN, and the general metabolic
ued until full enteral feedings are achieved. (5) In ex- demands of the neonate. In general, if PN is used, the daily
tremely preterm neonates receiving full EN, ESPGHAN energy requirement is reduced by 10% to 30%. (10)
recommends 4 g/kg per day of enteral protein for ade-
quate growth, but a higher intake of up to 4.5 g/kg per Sources of Energy
day may be needed for optimal weight gain. (2) Typical Neonates able to enterally feed with human milk or for-
preterm formulas contain 2.6 g of protein per 100 mL, mula receive 10 to 14 g/kg per day of carbohydrates, which
which corresponds to a protein intake of 3.9 to 4.7 g/kg per is usually sufficient for growth. In those requiring PN, a
day at enteral intakes of 150 to 180 mL/kg per day. Human GIR ranging from 2.5 mg/kg per minute on day 1 to a
milk, particularly donor human milk, is low in protein un- maximum of 10 mg/kg per minute is recommended. (11)
less provided in volumes greater than 200 mL/kg per day; Hyperglycemia should be anticipated and managed ac-
thus, multi-nutrient fortification is necessary to achieve the cordingly. Neonates who exceed the GIR requirements re-
desired protein concentrations of 4.5 g/kg per day. (2) quire further evaluation.
Lipids provide preterm infants with most of their en- Protein requirements are lower in term than in preterm
ergy requirements, and consequently, a relatively high die- neonates. (8) Human milk and infant formulas provide
tary lipid content to supply 36% to 67% of energy intake sufficient protein (1.5–2.5 g/kg per day) for a term neonate
is recommended. (6) In preterm infants with a daily en- if consumed in amounts sufficient to meet energy needs.
ergy intake of 110 kcal/kg, lipid intake is approximately 4.1 Human milk is whey predominant, though the ratio of
Vol. 25 No. 1 JANUARY 2024 e27
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
whey to casein varies over time, ranging from 90:10 in of 2 large randomized controlled trials. (19)(20) An initial
the colostrum to 60:40 in mature milk. Whey is more eas- CRRT dose prescription of 25 to 30 mL/kg per hour is rec-
ily digestible and absorbable and adds a lower solute load ommended in adults. (21) In pediatric patients, a corre-
on the kidney. (12) Most commercially available formulas sponding dose of 2,000 mL/1.73m2 per hour is used. (22)
designed for term infants have a whey-to-casein ratio of While the conversion from a weight-based dose in adults
60:40. Proteins when given by PN may be started at 1.5 g/kg matches well with a body surface area (BSA)–based dose
per day. In hospitalized neonates, catabolism is often present, in older children, the nonlinear relationship between weight
and protein requirements are often increased up to 3 g/kg and BSA results in a disproportionately higher dose in neo-
per day. Protein utilization for growth, and not energy, is best nates and infants. (23) The impact of this difference in
when 30 to 40 kcal/g of protein is provided. (13) Most paren- CRRT dosing on nutritional needs in neonates has not been
teral protein solutions contain essential amino acids. well studied.
Approximately 5 g/kg per day of fat is needed in term Malnutrition is extremely common in patients with
neonates. Both human milk and infant formula provide a AKI receiving CRRT, with a reported incidence of 30% to
sufficient quantity of lipids. In term infants, parenteral 55%. (24) One multicenter study of children receiving
lipid intake should not exceed 4 g/kg per day. (14) Formu- CRRT showed that many were receiving insufficient pro-
lations that have essential fatty acids should be used. tein. (25) Additionally, CRRT may result in the depletion
(loss) of important solutes necessary for growth and devel-
ACUTE KIDNEY INJURY opment. This loss of proteins appears to be dependent on
Pathophysiology of Compromised Nutrition the dialysis dose. (26) A study in adults showed that pa-
Critical illness is often characterized by a catabolic state, tients with severe AKI, even without CRRT, had micronu-
with muscle protein breakdown and lipolysis, and deterio- trient concentrations below the reference range that were
ration in nutritional status is common. (15)(16) This is am- not different from those receiving CRRT. (27) These data
plified in neonates who have high nutrition requirements suggest that micronutrient absorption and utilization may
for their rapid rate of growth yet have limited nutrition re- be impaired in AKI in general. (27) Zappitelli and col-
serves. In addition to protein catabolism, other metabolic leagues evaluated nutritional losses in children receiving
abnormalities seen in AKI include altered amino acid me- CRRT and reported that 10% of total amino acid intake was
tabolism, peripheral insulin resistance, and induction of a lost during CRRT. (28) Furthermore, patients had a nega-
proinflammatory state that can affect immune function. tive nitrogen balance despite protein provision of approxi-
(17) Hyperglycemia due to peripheral insulin resistance mately 2 g/kg per day. (28) The authors also identified loss
and hepatic gluconeogenesis is common. While these fac- of folate and selenium during CRRT and suggested that
tors lead to a loss of existing protein and energy stores, op- standard supplementation of these micronutrients may not
timal nutrition delivery in neonates and infants with AKI be sufficient. A second study from the same center (n515,
is also exceptionally challenging. Pathologic positive fluid median age of 2 years) found that CRRT resulted in a me-
balance and fluid overload present in the setting of AKI of- dian loss of 14.6% of prescribed protein. (26) Finally, in a
ten require fluid restriction, which makes it harder to pro- single-center prospective observational study of 174 children
vide optimal nutrition. As a result, critically ill neonates receiving CRRT (median age of 18 months), 56% had acute
(and infants and older children) with AKI are frequently protein-energy wasting, defined as weight greater than the
underfed and more likely to be fasting, thus placing them 3rd percentile. (29) Only protein energy wasting was associ-
at even greater risk for malnutrition. (18) Finally, AKI is ated with mortality in this cohort. (29) Unfortunately, most
also associated with electrolyte derangements such as hy- of these studies include older children. There is limited
ponatremia, hyperkalemia, hyperphosphatemia, and meta- data on how these losses affect neonates who are in a criti-
bolic acidosis, all of which may necessitate modifications cal stage of growth and development.
to EN or PN. Feeding modality may also be a barrier to the provision
A small subset of patients with severe AKI may require of adequate nutrition in neonates with severe AKI, includ-
RRT, either as continuous RRT (CRRT) or peritoneal dial- ing those who require CRRT. A recent single-center study
ysis (PD). Despite the advances in technology, CRRT pre- evaluated 41 patients (median age of 9 years) receiving
scriptions in neonates, infants, and children have been CRRT. (30) Approximately 50% of patients received a com-
largely extrapolated from adults. In adults, the CRRT dose bination of PN and EN, with 34% receiving exclusively PN
at initiation of therapy was standardized after the results and 12% receiving only EN. The percentage of time meeting
e28 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
protein goals by feeding modality was 65% for EN1PN, the assessment of intravascular volume as well as extravas-
34.6% for PN only, and 27.6% for EN only. Interestingly, cular fluid (such as free intra-abdominal fluid and pleural
when these patients were weaned to EN only from PN1EN, effusion). (33)
the average percentage of time protein goals that were met de-
creased to 20%. While this study included older children, Nutrition Prescription in Neonates with AKI
these data highlight that children with AKI are at significantly The diagnosis of AKI has several potential implications for
increased nutritional risk when they transition from PN to EN. nutrition assessment and provision in neonates. The goal
is to provide appropriate nutrition while achieving fluid
Estimating Nutritional Needs in AKI homeostasis and avoiding dehydration or progressive volume
Estimating nutritional needs in critically ill neonates, in- overload. This goal can be difficult to achieve, especially if
fants, and children is very challenging. Direct measures of fluid restriction is necessary in the setting of oliguria or an-
energy expenditure and indirect calorimetry are not usu- uria. (34) The inability to provide nutrition secondary to fluid
ally available in clinical practice, and resting energy expen- restriction in AKI is an indication of RRT.
diture (REE) may be calculated using the World Health Energy expenditure in neonates with AKI may depend
Organization or the Schofield equations. (24) In critically ill more on the underlying disease, the preexisting nutritional
children, in the acute phase, caloric needs equal REE. (24) status, and the coexistence of acute and chronic comorbid-
The subsequent recovery phase should incorporate addi- ities. Based on the phase of AKI, the caloric prescription
tional energy requirements for rehabilitation and growth. must account for rehabilitation and growth. It may also
Early and repeated nutrition screening and assessment need to be modified to account for RRT-related net gain or
are recommended to identify children at risk for malnutri- loss of energy. Both CRRT and PD provide other sources
tion. (24) While there are no screening tools specific to of calories for nutrition. For CRRT, this includes citrate
AKI in neonates, accurate anthropometric measurements (3 kcal/g) from the anticoagulant used, and lactate (3.62 kcal/g)
should be obtained early and repeated at least weekly. and glucose from the dialysis fluid. PD provides calories from
These include euvolemic weight, recumbent length, head the glucose in the dialysis fluid (7–9 kcal/kg per day), which
circumference, and assessment of muscle and fat distribu- should be a part of the prescription. (35) Critically ill neonates
tion by measuring middle upper arm circumference and with AKI may require higher protein intake to limit negative
skinfold thickness. Reference values for arm circumfer- protein balance. Protein restriction to lower blood urea ni-
ence and skinfold thickness from the World Health Orga- trogen levels or delay RRT initiation is not recommended.
nization are only available after 3 months of age, but there (21)(35) Protein losses may occur during PD, on the order
are other smaller studies that have looked at these meas- of approximately 0.1 to 0.28 g/kg per day, and additional
urements in neonates. (31)(32) Fluid balance may interfere protein intake of at least 0.1 g/kg per day should be pro-
with the use of weight as a marker of nutritional status in vided to infants on PD. (35) Table 2 summarizes the sug-
those with AKI. Other measures such as net fluid balance, gested dietary intake (SDI) in healthy term neonates and
bioimpedance electrical analysis, or point-of-care ultraso- those treated with RRT.
nography may help delineate volume status. Bioimpe- It is also important to appropriately supplement micro-
dance analysis can estimate body composition (particularly nutrients (vitamins, trace elements, and carnitine). Sup-
total body water and lean mass) by measuring opposition plemental vitamin A should be avoided in all neonates
to electrical flow through tissues with the current and de- with AKI because impaired kidney function is associated
tection electrodes placed on the infant’s wrist and ankle. with reduced excretion of vitamin A, as well as elevated lev-
(32) However, the prediction equations using this method els of retinol, both of which may result in hypercalcemia and
are less accurate for neonates as compared to older chil- hypervitaminosis A. (24) Those requiring RRT may need
dren. (32) Point-of-care ultrasonography may be used for supplemental water-soluble vitamins, trace elements (copper,
Table 2. Modifications to the Suggested Dietary Intake of Calories and Proteins in Healthy Term Neonates and
Those on Chronic Dialysis
Energy Protein
Term neonate 93–107 kcal/kg per day 1.5–2.5 g/kg per day
Peritoneal dialysis Deduct 7–9 kcal/kg per day Add 0.15–0.3 g/kg per day
Hemodialysis Deduct !3 kcal/kg per day Add 0.1 g/kg per day
Vol. 25 No. 1 JANUARY 2024 e29
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
zinc, selenium), and carnitine. There are no data to recom- hypothalamic neuronal circuits that control appetite. (37)
mend routine measurement of serum concentrations of mi- There are also higher circulating concentrations of cyto-
cronutrients unless a deficiency or toxicity is suspected based kines, such as leptin, tumor necrosis factor a, and inter-
on clinical signs. leukins 1 and 6, which further lead to cachexia. (37)
Additional electrolyte abnormalities seen in AKI may
require modification of the nutrition prescription. Normal Estimating Nutritional Needs in CKD
values of plasma potassium in neonates are higher com- Regular assessment by a pediatric dietitian experienced in
pared to levels in older infants, children, and adults, and caring for neonates with CKD is imperative, and the Kidney
appropriate interpretation requires the use of age-based Disease Outcomes Quality Initiative (KDOQI) clinical prac-
normative values. (36) For infants with AKI and hyperkale- tice guideline includes specific recommendations for regular
mia, human milk, which is naturally low in potassium evaluations of growth and nutritional status in these neo-
compared to standard infant formulas, may be used. Alter- nates. These recommendations include a review of dietary
native approaches include decanting milk with potassium- intake and assessments of length for age, estimated euvole-
binding resins, use of low-potassium infant formulas, or a mic or “dry” weight and weight for age, body mass index
brief period of discontinuing EN and supplementing with (BMI) for height/length for age, and head circumference.
IV fluids or PN without potassium. If the patient is already (38) This is echoed by the more recent Pediatric Renal Nutri-
receiving nutrition exclusively via IV fluids or PN, potassium tion Taskforce (PRNT) (39) guidelines that recommended as-
should be removed from these fluids until hyperkalemia is sessment of these parameters at least twice as frequently as
corrected. The enteral or rectal use of sorbitol-based resins they would be performed in a healthy neonate, although
such as sodium polystyrene should be avoided in neonates more frequent assessments may be needed in neonates with
because of the risk of intestinal perforation and obstruction. severe disease or complications (polyuria, growth delay, de-
(36) Hyperphosphatemia may require phosphate restriction creasing/low BMI, comorbid pathologies impacting growth
and transition to a low-phosphorous renal infant formula or or nutrient intake, and recent acute medical status changes).
treatment with a calcium-based phosphate binder. Hypophos- (38) For preterm infants born between 32 and 37 weeks’ ges-
phatemia is common during CRRT, and phosphorus supple- tation, PRNT recommends plotting anthropometric measure-
mentation is often needed to avoid complications such as ments for both gestational and chronologic age for the first
respiratory muscle weakness, myocardial dysfunction, and en- year after birth, and for those born before 32 weeks’ gesta-
cephalopathy. Phosphate is commonly provided as an additive tion, these measurements should be obtained through 2 years
to CRRT solutions, though in recent years, commercially of age. (39) Consideration of the underlying pathology of
available phosphate-containing CRRT solutions have become CKD is also important when nutritional prescriptions are de-
available. Patients often need additional supplementation ei- termined; infants with congenital nephrotic syndrome, for ex-
ther orally or in the PN. Table 3 summarizes the electrolyte ample, experience excessive protein losses and may require
requirements in healthy neonates and those with AKI. additional protein/amino acid supplementation beyond that of
infants with CKD secondary to other causes. Careful assess-
CHRONIC KIDNEY DISEASE ment of body weight and fluid balance is of paramount im-
Pathophysiology of Compromised Nutrition portance given that the prescription of nutrition is often based
The altered metabolic milieu found in patients with AKI is on body weight and both volume overload and that depletion
also seen in those with CKD. Additional changes include can occur in neonates with CKD. Dry weight should be fre-
anorexia secondary to abnormalities in the hormonal and quently reassessed by evaluating body weight along with the
Table 3. Electrolyte Requirements in Healthy Term Neonates and Typical Changes seen in Patients with Acute
Kidney Injury Compared to Normal
Healthy Term Neonates Term infants With Acute Kidney Injury
No RRT iHD PD CRRT
Sodium (mEq/kg/day) 2-5 May decrease May decrease May decrease May decrease
Potassium (mEq/kg/day) 2-4 Lower Unchanged /lower Unchanged /lower Higher
Calcium (mMol/kg/d) 0.25-2 Higher Higher Higher Higher
Phosphorus (mMol/kg/d) 0.5-2 Lower Unchanged /lower Unchanged /lower Higher
Magnesium (mMol/kg/d) 0.15-0.25 Unchanged Unchanged Unchanged Higher
Acetate/bicarbonate As needed Higher Unchanged Unchanged Lower
RRT, renal replacement therapy; iHD, intermittent hemodialysis; PD, peritoneal dialysis; CRRT, chronic RRT
e30 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
presence or absence of edema, blood pressure, laboratory val- Neonates with CKD requiring RRT warrant special con-
ues, and dietary intake. (38) Individualized counseling and in- sideration regarding their nutritional needs. Some of these
terventions, as well as frequent reevaluations and potential needs are similar to those discussed for AKI. Adequacy of
modifications to nutrition plans of care, may be needed. This nutrition provision is of particular importance during PD
requires the coordinated efforts of a dietitian with expertise in catheter placement because malnutrition with subsequent
pediatric and renal nutrition, as well as a multidisciplin- abdominal muscle wasting can lead to poor stability of the
ary team including the child, caregivers, and the pediatric catheter, potential leakage, and impaired wound healing.
nephrology team. (41) In children receiving PD, the energy intake from
the dialysate should be considered to range from 7 to
9 kcal/kg per day depending on the dextrose concentra-
Nutrition Prescription in Neonates with CKD
tion of the dialysate, the time on dialysis, number of
KDOQI recommends centering nutritional care to achieve
cycles, dwell times, and the peritoneal membrane trans-
the following goals:
porter status. If there is excessive weight gain in these
1. “maintenance of an optimal nutritional status (ie,
children, this energy source needs to be taken into con-
achievement of normal pattern of growth and body com-
sideration. Dialysate protein loss should be accounted
position by intake of appropriate amounts and types of
for, and the recommended protein intake is higher than the
nutrients),
SDI for neonates managed conservatively, with an addi-
2. avoidance of uremic toxicity, metabolic abnormalities,
tional 0.15 to 0.3 g/kg per day recommended on PD and
and malnutrition, and
0.1 g/kg per day on intermittent hemodialysis (Table 3).
3. reduction of the risk of chronic morbidities and mortal-
(40) Neonates on PD frequently experience vomiting, poor
ity in adulthood.”(38)
appetite and oral intake, and delayed gastric emptying,
which can exacerbate nutritional deficiencies and losses.
More recently Shaw and colleagues from PRNT pro-
(41) Adequate growth and even catch-up growth can be
vided an update on energy and protein requirements for
achieved with appropriate nutrition provision in neonates
children with CKD stages 2 to 5 and on dialysis. (35) Given
with CKD with or without RRT.
the varying national and international terms and defini-
The intake of calcium should be maintained between
tions for energy and protein requirements, PRNT intro-
100% and 200% of SDI. The calcium intake from medica-
duced the term “suggested dietary intake” (SDI) for their
tion and dialysate should be considered. The dietary phos-
recommendations. In CKD stages 2 to 5, initial prescrip-
phate intake should be within the SDI. Human milk and
tions for nutrition should approximate that of healthy chil-
whey-dominant formula are low in phosphate. (42) Potas-
dren of the same chronologic age, and when weight gain sium intake should be based on renal function and serum
is suboptimal, the prescription should be adjusted toward potassium levels. Human milk has a low potassium con-
the higher end of the SDI. In infants at risk for obesity, tent. In neonates with hyperkalemia, human milk may be
the nutrition should be adjusted to achieve appropriate substituted with a low-potassium formula. In formula-fed
weight gain without compromising the delivery of protein, infants, the potassium-binding resin can be added to the
fat, carbohydrates, and macro- and micronutrients. In neo- formula and decanted. (43)
nates, human milk is preferred, with appropriate fortifica- While the preferred route for the provision of nutrition is
tion, when necessary, in the setting of fluid restriction, or oral, enteral tube feeding via a nasogastric tube or gastrostomy
when additional calories are required without increasing device may be occasionally needed. PRNT recommends sup-
total volume. A whey-dominant formula is recommended plemental or exclusive enteral tube feeding in children with
when supplemental feedings are required. Methods of CKD who are unable to meet their nutritional requirements
fortification of human milk with whey-based formulas orally. (35) Despite these recommendations, various studies
have been described elsewhere. (40) A feeding volume of have shown limited use of enteral tube feeding outside North
150 mL/kg per day may be sufficient to meet the nutri- America and some European countries. (44)(45)(46) For short-
tional needs of an infant with CKD. However, an infant term enteral feeding needs, a nasogastric tube is preferred,
with polyuria with a higher-than-normal fluid require- whereas a gastrostomy device is preferred for long-term use.
ment may need additional fluid given either as free water (47) PRNT provides additional clinical practice recommenda-
in between feedings or mixed in to prepare dilute formula. tions for the optimal delivery of nutrition via enteral tube feed-
(40) ing to children with CKD stages 2 to 5 and on dialysis. (47)
Vol. 25 No. 1 JANUARY 2024 e31
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
CONCLUSION 5. van den Akker CHP, Saenz de Pipaonl M, van Goudoever JB.
Proteins and amino acids. In: Koletzko B, Cheah FC, Domellof M,
Neonates with kidney dysfunction, be it AKI or CKD, have Poindexter B, Vain N, van Goudoever J, eds. Nutritional Care of
unique nutrition needs. It is important to be aware of Preterm Infants Scientific Basis and Practice Guidelines World Review of
their nutritional requirements, know how to assess them Nutrition and Dietetics. Vol. 122. Basel: Karger; 2021:75–88
and be able to provide appropriate nutrition, especially as 6. Koletzko B, Lapillonne A. Lipid requirements of preterm infants. In:
Koletzko B, Cheah FC, Domellof M, Poindexter B, Vain N, van
outlined by PRNT. Based on the type of kidney dysfunction
Goudoever J, eds. Nutritional Care of Preterm Infants Scientific Basis
(acute or chronic) and the need for RRT (CRRT, intermit- and Practical Guidelines World Review of Nutrition and Dietetics. Basel:
tent hemodialysis, or PD), the needs and losses of macro- Karger; 2021:89–102
and micronutrients and trace minerals may vary, necessitat- 7. Meek JY, Noble L; Section on Breastfeeding. Policy statement:
ing periodic laboratory monitoring and adjustment. breastfeeding and the use of human milk. Pediatrics. 2022;150(1):
e2022057988
8. Hermoso M, Tabacchi G, Iglesia-Altaba I, et al. The nutritional
requirements of infants: towards EU alignment of reference values:
American Board of Pediatrics the EURRECA network. Matern Child Nutr. 2010;6(suppl 2):55–83
Neonatal-Perinatal Content doi: 10.1111/j.1740-8709.2010.00262.x
Specifications 9. Working Group of Pediatrics Chinese Society of Parenteral and
Enteral Nutrition; Working Group of Neonatology Chinese Society
• Know the differences in the nutritional of Pediatrics; Working Group of Neonatal Surgery Chinese Society
composition of human milk and infant formula. of Pediatric Surgery. CSPEN guidelines for nutrition support in
neonates. Asia Pac J Clin Nutr. 2013;22(4):655–663
• Know the importance of protein and nonprotein
10. Joosten K, Embleton N, Yan W, Senterre T; ESPGHAN/ESPEN/
nutrients in achieving optimal utilization of energy
ESPR/CSPEN Working Group on Pediatric Parenteral Nutrition.
and nitrogen. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral
• Know the etiology of electrolyte abnormalities in nutrition: energy. Clin Nutr. 2018;37(6 Pt B):2309–2314
the neonate. 11. Mesotten D, Joosten K, van Kempen A, Verbruggen S; ESPGHAN/
• Know the impact of renal dysfunction on water ESPEN/ESPR/CSPEN Working Group on Pediatric Parenteral
Nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric
requirements.
parenteral nutrition: carbohydrates. Clin Nutr. 2018;37(6 Pt B):
• Know how to manage electrolyte abnormalities in 2337–2343
the neonate. 12. L€
onnerdal B. Nutritional and physiologic significance of human
• Know the management of renal failure in the milk proteins. Am J Clin Nutr. 2003;77(6):1537S–1543S
neonate, including indications for and 13. Mustapha M, Wilson KA, Barr S. Optimising nutrition of preterm
and term infants in the neonatal intensive care unit. Paediatr Child
complications of the use of hemofiltration,
Health. 2021;31(1):38–45
peritoneal dialysis, and hemodialysis.
14. Lapillonne A, Fidler Mis N, Goulet O, van den Akker CHP, Wu J,
• Know the potential adverse effects of Koletzko B; ESPGHAN/ESPEN/ESPR/CSPEN Working Group on
pharmacologic use of fat-soluble vitamins. Pediatric Parenteral Nutrition. ESPGHAN/ESPEN/ESPR/CSPEN
• Know the medical indications for the use of guidelines on pediatric parenteral nutrition: lipids. Clin Nutr.
2018;37(6 Pt B):2324–2336
nonstandard infant formulas to meet the needs of
15. Preiser JC, Ichai C, Orban JC, Groeneveld AB. Metabolic response
infants with special health problems. to the stress of critical illness. Br J Anaesth. 2014;113(6):945–954
16. Valla FV, Baudin F, Gaillard Le Roux B, et al. Nutritional status
deterioration occurs frequently during children’s ICU stay. Pediatr
Crit Care Med. 2019;20(8):714–721
References 17. Fiaccadori E, Sabatino A, Barazzoni R, et al. ESPEN guideline on
1. Koletzko B, Cheah FC, Domell€ of M, van Goudoever JB, Poindexter clinical nutrition in hospitalized patients with acute or chronic
BB, Vain N. Scientific basis and practical application of nutritional kidney disease. Clin Nutr. 2021;40(4):1644–1668
care for preterm infants. World Rev Nutr Diet. 2021;122:XIII–XIV 18. Kyle UG, Akcan-Arikan A, Orellana RA, Coss-Bu JA. Nutrition
2. Embleton ND, Jennifer Moltu S, Lapillonne A, et al. Enteral support among critically ill children with AKI. Clin J Am Soc
nutrition in preterm infants (2022): a position paper from the Nephrol. 2013;8(4):568–574
ESPGHAN Committee on Nutrition and Invited Experts. J Pediatr 19. Palevsky PM, Zhang JH, O’Connor TZ, et al; VA/NIH Acute Renal
Gastroenterol Nutr. 2023;76(2):248–268 Failure Trial Network. Intensity of renal support in critically ill
3. Hay WW Jr. Nutritional support strategies for the preterm infant in patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20
the neonatal intensive care unit. Pediatr Gastroenterol Hepatol Nutr. 20. Bellomo R, Cass A, Cole L, et al; RENAL Replacement Therapy
2018;21(4):234–247 Study Investigators. Intensity of continuous renal-replacement
4. Embleton ND, van den Akker CHP. Protein intakes to optimize therapy in critically ill patients. N Engl J Med. 2009;361(17):
outcomes for preterm infants. Semin Perinatol. 2019;43(7):151154 1627–1638
e32 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
21. KDIGO. Clinical Practice Guideline for Acute Kidney Injury. Kidney 35. Shaw V, Polderman N, Renken-Terhaerdt J, et al. Energy and
Int Suppl. 2012; (2):1–138 protein requirements for children with CKD stages 2-5 and on
22. Maxvold NJ, Smoyer WE, Custer JR, Bunchman TE. Amino acid dialysis-clinical practice recommendations from the Pediatric Renal
loss and nitrogen balance in critically ill children with acute renal Nutrition Taskforce. Pediatr Nephrol. 2020;35(3):519–531
failure: a prospective comparison between classic hemofiltration and 36. Bonilla-F"elix M. Potassium regulation in the neonate. Pediatr
hemofiltration with dialysis. Crit Care Med. 2000;28(4):1161–1165 Nephrol. 2017;32(11):2037–2049
23. Ricci Z, Guzzi F, Tuccinardi G, Romagnoli S. Dialytic dose in 37. Mak RH, Cheung W, Cone RD, Marks DL. Orexigenic and
pediatric continuous renal replacement therapy patients. Minerva anorexigenic mechanisms in the control of nutrition in chronic
Pediatr. 2016;68(5):366–373 kidney disease. Pediatr Nephrol. 2005;20(3):427–431
24. Vega MRW, Cerminara D, Desloovere A, et al. Nutritional 38. KDOQI Work Group. 2008 update. Executive summary. Am J
management of children with acute kidney injury-clinical practice Kidney Dis. 2009;53(3 suppl 2):S11–S104
recommendations from the Pediatric Renal Nutrition Taskforce.
39. Nelms CL, Shaw V, Greenbaum LA, et al. Assessment of nutritional
Pediatr Nephrol. 2023;38(11):3559–3580
status in children with kidney diseases-clinical practice
25. Zappitelli M, Goldstein SL, Symons JM, et al; Prospective Pediatric recommendations from the Pediatric Renal Nutrition Taskforce.
Continuous Renal Replacement Therapy Registry Group. Protein Pediatr Nephrol. 2021;36(4):995–1010
and calorie prescription for children and young adults receiving
40. Shaw V, Anderson C, Desloovere A, et al. Nutritional management
continuous renal replacement therapy: a report from the Prospective
of the infant with chronic kidney disease stages 2-5 and on dialysis.
Pediatric Continuous Renal Replacement Therapy Registry Group.
Pediatr Nephrol. 2023;38(1):87–103
Crit Care Med. 2008;36(12):3239–3245
41. Spector BL, Misurac JM. Renal replacement therapy in neonates.
26. Lion RP, Vega MR, Smith EO, et al. The effect of continuous
NeoReviews. 2019;20(12):e697–e710
venovenous hemodiafiltration on amino acid delivery, clearance, and
removal in children. Pediatr Nephrol. 2022;37(2):433–441 42. McAlister L, Pugh P, Greenbaum L, et al. The dietary
management of calcium and phosphate in children with CKD
27. Ostermann M, Summers J, Lei K, et al. Micronutrients in critically
stages 2-5 and on dialysis-clinical practice recommendation
ill patients with severe acute kidney injury - a prospective study. Sci
from the Pediatric Renal Nutrition Taskforce. Pediatr Nephrol.
Rep. 2020;10(1):1505
2020;35(3):501–518
28. Zappitelli M, Juarez M, Castillo L, Coss-Bu J, Goldstein SL.
43. Desloovere A, Renken-Terhaerdt J, Tuokkola J, et al. The dietary
Continuous renal replacement therapy amino acid, trace metal and
management of potassium in children with CKD stages 2-5 and
folate clearance in critically ill children. Intensive Care Med.
on dialysis-clinical practice recommendations from the Pediatric
2009;35(4):698–706
Renal Nutrition Taskforce. Pediatr Nephrol. 2021;36(6):
29. Castillo A, Santiago MJ, L"
opez-Herce J, et al. Nutritional status and 1331–1346
clinical outcome of children on continuous renal replacement
44. Rees L, Azocar M, Borzych D, et al; International Pediatric
therapy: a prospective observational study. BMC Nephrol. 2012;13:125
Peritoneal Dialysis Network (IPPN) registry. Growth in very young
30. Wong Vega M, Juarez Calderon M, Tufan Pekkucuksen N, Srivaths children undergoing chronic peritoneal dialysis. J Am Soc Nephrol.
P, Akcan Arikan A. Feeding modality is a barrier to adequate protein 2011;22(12):2303–2312
provision in children receiving continuous renal replacement
45. Schaefer F, Benner L, Borzych-Du_załka D, et al; International
therapy (CRRT). Pediatr Nephrol. 2019;34(6):1147–1150
Pediatric Peritoneal Dialysis Network (IPPN) Registry. Global
31. Pereira-da-Silva L, Virella D, Fusch C. Nutritional assessment in variation of nutritional status in children undergoing chronic
preterm infants: a practical approach in the NICU. Nutrients. peritoneal dialysis: a longitudinal study of the International Pediatric
2019;11(9):1999 Peritoneal Dialysis Network. Sci Rep. 2019;9(1):4886
32. Demerath EW, Fields DA. Body composition assessment in the
46. Sharma S, Sinha A, Malik R, Bagga A. Gastrostomy tube feeding in
infant. Am J Hum Biol. 2014;26(3):291–304 Indian children with advanced chronic kidney disease. Indian J
33. Singh Y, Tissot C, Fraga MV, et al. International evidence-based Pediatr. 2023;90(4):400–402
guidelines on point of care ultrasound (POCUS) for critically ill
47. Rees L, Shaw V, Qizalbash L, et al; Pediatric Renal Nutrition
neonates and children issued by the POCUS Working Group of the
Taskforce. Delivery of a nutritional prescription by enteral
European Society of Paediatric and Neonatal Intensive Care
tube feeding in children with chronic kidney disease stages
(ESPNIC). Crit Care. 2020;24(1):65 2-5 and on dialysis-clinical practice recommendations from the
34. Nada A, Bonachea EM, Askenazi DJ. Acute kidney injury in the Pediatric Renal Nutrition Taskforce. Pediatr Nephrol. 2021;
fetus and neonate. Semin Fetal Neonatal Med. 2017;22(2):90–97 36(1):187–204
Vol. 25 No. 1 JANUARY 2024 e33
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
NEOREVIEWS QUIZ
NEO
QUIZ
1. A preterm infant of 31 week’s gestation is starting enteral feeds. The NICU
team is working on calculating the caloric density of feeds to ensure
adequate growth. Which of the following statements describes an
appropriate daily total energy intake for this preterm infant?
A. 60 kcal/kg per day.
B. 75 kcal/kg per day.
C. 90 kcal/kg per day.
D. 120 kcal/kg per day
E. 150 kcal/kg per day.
REQUIREMENTS: Learners can
2. You are caring for an infant born at 25 week’s gestation who is advancing take NeoReviews quizzes and
enteral feeds. Previous studies have demonstrated that proteins are key claim credit online only at:
structural components of all human cells, vital for adequate growth, and a https://publications.aap.org/
main driver of lean body mass growth. What is the recommended amount of neoreviews.
enteral protein intake for adequate growth in an extremely preterm infant?
To successfully complete 2024
A. 11.5 g/kg per day NeoReviews articles for AMA PRA
B. 2 to 2.5 g/kg per day. Category 1 Credit™, learners
must demonstrate a minimum
C. 3 to 3.5 g/kg per day.
performance level of 60% or
D. 4 to 4.5 g/kg per day. higher on this assessment. If
E. 5 to 5.5 g/kg per day. you score less than 60% on the
assessment, you will be given
3. You are taking care of a critically ill term neonate with acute kidney injury additional opportunities to
(AKI). Infants with this condition are often in a catabolic state with muscle answer questions until an
protein breakdown and lipolysis. In addition to increased protein catabolism, overall 60% or greater score is
other metabolic abnormalities can occur in infants with AKI. All of the achieved.
following metabolic derangements can be observed in these patients except:
This journal-based CME activity
A. Altered amino acid metabolism. is available through Dec. 31,
B. Hypoglycemia. 2026, however, credit will be
recorded in the year in which
C. Impaired immune function.
the learner completes the quiz.
D. Peripheral insulin resistance.
E. Proinflammatory state.
4. AKI is a common complication in neonates with critical illness and can be
challenging to manage. Pathologic fluid balance and fluid overload often
require fluid restriction, making it difficult to provide optimal nutrition. In
addition to fluid overload AKI can contribute to each of the following 2024 NeoReviews is approved
for a total of 30 Maintenance of
electrolyte derangements EXCEPT:
Certification (MOC) Part 2
A. Hypercalcemia. credits by the American Board
B. Hyperkalemia. of Pediatrics (ABP) through the
AAP MOC Portfolio Program.
C. Hyponatremia.
NeoReviews subscribers can
D. Hyperphosphatemia. claim up to 30 ABP MOC Part 2
E. Metabolic acidosis. points upon passing 30 quizzes
(and claiming full credit for
each quiz) per year. Subscribers
can start claiming MOC credits
as early as October 2024. To
learn how to claim MOC points,
go to: https://publications.aap.
org/journals/pages/moc-credit.
e34 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
5. An infant of 37 week’s gestation with AKI is requiring continuous renal
replacement therapy (CRRT). A common complication of CRRT is the removal
of important solutes necessary for growth and development. Of the patients
receiving CRRT, what percentage experience malnutrition and poor growth?
A. 10%
B. 25%.
C. 45%.
D. 60%.
E. 75%.
Vol. 25 No. 1 JANUARY 2024 e35
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
ARTICLE
Common Clinical Scenarios of Systemic
Hypertension in the NICU
Sheema Gaffar, MD, MS,*† Rangasamy Ramanathan, MD,* Molly Crimmins Easterlin, MD, MS†
*Division of Neonatology, Department of Pediatrics, Los Angeles General Medical Center, Keck School of Medicine, University of Southern California, Los
Angeles, CA
†
Division of Neonatology, Fetal and Neonatal Institute, Children’s Hospital Los Angeles, Keck School of Medicine, University of Southern California, Los
Angeles, CA
PRACTICE GAPS
The diagnostic criteria and treatment of neonatal hypertension are
inconsistent. Due to an insufficient number of published studies and a lack
of evidence on the long-term effects of hypertension and on the safety and
efficacy of antihypertensive medications, guidelines for the treatment of
hypertension in neonates and infants are lacking. Long-term effects of
neonatal hypertension are also underrecognized, resulting in variable
practices for monitoring and follow-up.
OBJECTIVES After completing this article, readers should be able to:
1. Identify hypertension in a neonate of any gestational age, initiate diagnostic
evaluation, and establish follow-up and long-term monitoring plan.
2. Recognize common clinical scenarios in which neonatal hypertension
can present in the NICU.
3. Describe antihypertensive therapeutic options in neonates.
ABSTRACT
AUTHOR DISCLOSURES Drs Easterlin,
Ramanathan, and Gaffar have disclosed Hypertension affects !1% to 3% of newborns in the NICU. However, the
no financial relationships relevant to this identification and management of hypertension can be challenging
article. This commentary does not because of the lack of data-driven diagnostic criteria and management
contain a discussion of an unapproved/
investigative use of a commercial
guidelines. In this review, we summarize the most recent approaches to
product/device. diagnosis, evaluation, and treatment of hypertension in neonates and
infants. We also identify common clinical conditions in neonates in whom
ABBREVIATIONS hypertension occurs, such as renal vascular and parenchymal disease,
bronchopulmonary dysplasia, and cardiac conditions, and address specific
ACE angiotensin-converting enzyme
AKI acute kidney injury considerations for the evaluation and treatment of hypertension in those
BPD bronchopulmonary dysplasia conditions. Finally, we discuss the importance of ongoing monitoring and
CoA coarctation of the aorta
long-term follow-up of infants diagnosed with hypertension.
ECMO extracorporeal membrane
oxygenation
IV intravenous
PDA patent ductus arteriosus INTRODUCTION
PMA postmenstrual age
SGA small for gestational age
Hypertension is relatively common in neonates in the NICU. Optimal management of
UAC umbilical arterial catheter neonatal hypertension is guided by the etiology of hypertension, determining when
e36 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
pharmacologic intervention is needed, which antihypertensive term infants, blood pressure rises by 1 to 2 mm Hg per day
medication to use, and treating the underlying cause. However, for the first 3 to 4 days after birth and then levels off. (1) In
identification and management of neonates with hypertension preterm infants, blood pressure is more labile and rises rap-
can be challenging because of a lack of data-driven diagnostic idly in the first few weeks of age, depending on the infant’s
criteria, limited studies on the long-term effects of hyperten- postmenstrual age (PMA), birthweight, and antenatal expo-
sion, and insufficient randomized controlled trials evaluating sure to medical conditions of pregnancy. (2)(3) In extremely
the safety and efficacy of antihypertensive medications. preterm infants, blood pressure may initially decrease for
In this review, we summarize the most recent approaches the first few hours after birth before increasing (Table 1).
to the diagnosis, evaluation, and treatment of hypertension (4)(5)
in neonates and infants. We also identify common clinical Due to this expected variability in blood pressure, it
conditions in the NICU in which hypertension occurs, in- can be difficult to strictly define normal blood pressure
and high blood pressure in the first weeks after birth. In
cluding renal vascular and parenchymal disease, bronchopul-
practice, for a preterm infant, the lowest acceptable mean
monary dysplasia (BPD), coarctation of the aorta (CoA),
arterial blood pressure in the first few days after birth is
postoperative pain and fluid shifts, and prematurity or growth
approximately equivalent to the gestational age in weeks,
restriction. We discuss specific management approaches for
as long as there is clinical evidence of good systemic per-
each cause of hypertension. Finally, we review the importance
fusion. (6)(7)(8) Once a neonate is 2 weeks old, the defini-
of ongoing monitoring and long-term follow-up of infants di-
tion of normal blood pressure is more standardized because
agnosed with hypertension.
some normative data exist. (9) The American Academy of
Pediatrics recommends using these blood pressure percen-
DEFINITION OF NEONATAL HYPERTENSION tiles to define normal blood pressure ranges for infants who
In neonates, blood pressure is variable in the first few days are older than 2 weeks’ chronologic age and 26 to 44 weeks’
after birth because of transitional physiology. Generally, in PMA (Table 2). (2)(9)(10)(11) Hypertension is typically defined
Table 1 . BP Values Obtained on the Newborn Day, 3 Days after Birth, and 5 Days after Birth
Newborn Day BP Day 3 BP Day 5 BP
Birthweight
(g) Average 1 0 th Pc 5 0 th Pc 9 0 th Pc Average 1 0 th Pc 5 0 th Pc 9 0 th Pc Average 1 0 th Pc 5 0 th Pc 9 0 th Pc
#1,000 SBP 56 40 55 72 60 46 59 77 62 49 62 77
DBP 35 21 36 48 40 27 38 56 39 29 38 51
MBP 43 27 41 56 48 35 46 64 47 36 47 59
1,001–1,500 SBP 56 43 55 71 65 53 65 78 67 54 68 79
DBP 35 24 36 45 42 32 42 53 43 33 43 54
MBP 43 31 43 56 51 40 51 61 52 41 52 62
1,501–2,000 SBP 59 48 58 70 65 54 65 77 68 57 68 81
DBP 35 26 35 44 42 33 41 52 42 32 42 53
MBP 44 34 44 54 51 42 51 60 52 42 52 64
2,001–2,500 SBP 60 50 59 72 68 58 68 79 73 60 73 86
DBP 37 27 36 46 44 35 44 53 45 35 44 57
MBP 46 36 45 55 53 44 53 62 56 45 55 69
2,501–3,000 SBP 67 54 64 82 71 59 71 84 73 63 74 83
DBP 43 30 41 58 45 34 44 56 43 35 42 54
MBP 52 40 50 68 55 44 54 67 55 45 54 66
3,001–3,500 SBP 69 56 69 82 74 62 73 87 75 63 75 88
DBP 42 32 42 52 47 37 46 58 46 37 45 56
MBP 52 42 52 63 58 46 57 70 57 48 57 68
3,501–4,000 SBP 70 59 70 81 75 62 74 86 80 69 80 91
DBP 42 32 41 52 48 37 47 59 48 39 48 59
MBP 53 43 52 64 58 46 58 70 60 50 60 71
4,001–4,500 SBP 71 58 70 88 75 62 75 88 77 61 78 90
DBP 43 35 42 54 47 36 45 59 46 36 44 58
MBP 53 43 52 66 58 45 58 71 58 45 58 69
Values are based on birthweight for preterm and term infants, based on a retrospective review of 629 infants in Hungarian NICUs. (4) Before
this study, the 1995 study by Zubrow et al (5) on 608 infants in Philadelphia NICUs was used for blood pressure norms in the first few days
after birth. BP5blood pressure, DBP5diastolic blood pressure, MBP5mean blood pressure, Pc5percentile, SBP5systolic blood pressure.
Reprinted with permission from Kiss et al. (4).
Vol. 25 No. 1 JANUARY 2024 e37
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
Table 2 . Blood Pressure Normative Values Obtained for Infants Older than 2 Weeks
Postconceptional Age 5 0 th Percentile 9 5 th Percentile 9 9 th Percentile
44 wk
SBP 88 105 110
DBP 50 68 73
MAP 63 80 85
42 wk
SBP 85 98 102
DBP 50 65 70
MAP 62 76 81
40 wk
SBP 80 95 100
DBP 50 65 70
MAP 60 75 80
38 wk
SBP 77 92 97
DBP 50 65 70
MAP 59 74 79
36 wk
SBP 72 87 92
DBP 50 65 70
MAP 57 72 71
34 wk
SBP 70 85 90
DBP 40 55 60
MAP 50 65 70
32 wk
SBP 68 83 88
DBP 40 55 60
MAP 48 62 69
30 wks
SBP 65 80 85
DBP 40 55 60
MAP 48 65 68
28 wks
SBP 60 75 80
DBP 38 50 54
MAP 45 58 63
26 wks
SBP 55 72 77
DBP 30 50 56
MAP 38 57 63
Infants are at 26 to 44 weeks’ PMA. These values, first published in 2012 by Dionne et al, (9) remain in use presently. DBP5diastolic blood
pressure, MAP5mean arterial pressure, SBP5systolic blood pressure.
Reprinted with permission from Kiss et al. (4).
as persistently elevated systolic, diastolic, or mean blood pres- best practice recommendations for blood pressure mea-
sure, greater than the 95th percentile, or any elevated surement in infants with an oscillometric device or with
blood pressure associated with signs or symptoms of or- auscultation (manual). (10)(12) Cuff size is selected in re-
gan damage. (6)(10) In the ambulatory setting, persistent lation to the size of the extremity used for measurement;
hypertension is defined as hypertension present in more the right upper arm is typically used for brachial artery
than 3 office visits. (10) In the intensive care unit, how- blood pressure measurement, as the left upper arm can
ever, persistent hypertension is vague and often deferred to be erroneous in cases of CoA. (10) Although the thigh
the clinician’s judgment. can be used for popliteal artery blood pressure measurement
The technique used in measuring blood pressure is im- in neonates, the values are not easily compared with pub-
portant as most oscillometric devices are not manufac- lished reference tables, which use the arm. (10) The length
tured specifically for the neonatal population. (12) The and width of the cuff should be 80% to 100% and 45% to
American Academy of Pediatrics, American Heart Associ- 70% of the arm circumference, respectively. (10) Measure-
ation, and National Institutes of Health have published ments should be taken while the infant is calm, laying in the
e38 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
supine position, 1.5 hours after the last feeding, and after the PATHOGENESIS
cuff has been in place for 15 minutes. A repeat blood pres- The pathogenesis of hypertension is unique to each clini-
sure assessment can be done if the first one is elevated, and cal condition.
the values can be averaged. (1)(2)(6)(10)(13) An umbilical ar-
terial catheter (UAC) provides direct, continuous intra-arterial UAC-associated Renovascular Hypertension
blood pressure measurements that are more accurate than UAC-associated thrombosis can result in a partially or
cuff measurements but also more invasive. completely occlusive aortic thrombus, from which a large
thrombus or smaller thrombi can embolize into the renal
parenchyma, causing hypertension. (12) Disruption of the
EPIDEMIOLOGY
vascular endothelium during placement of the catheter it-
While the incidence of hypertension varies slightly based
self may also lead to the formation of microemboli, which
on the definition of hypertension used, it occurs in !1%
may deposit in the renal artery, decreasing perfusion to
to 3% of infants hospitalized in the NICU. (2)(6) It is the kidney, stimulating renin and aldosterone production,
more common in the NICU population than in healthy and causing hypertension secondary to increased sodium
term newborns where the incidence is !0.2%. (9)(14) Ap- reabsorption and water retention. (20) These microemboli
proximately half (52.8%–57.7%) of neonates diagnosed may be too small to detect on ultrasonography. (12)(14)(16)
with hypertension in the NICU receive pharmacologic treat- Other renovascular abnormalities such as renal artery
ment with at least 1 antihypertensive medication. (13)(15) stenosis, renal vein thrombosis, and fibromuscular dyspla-
Risk factors for neonatal hypertension include antenatal sia are rare but possible causes of neonatal hypertension,
factors such as exposure to hypertension of pregnancy, ex- especially in the setting of other common risk factors for
posure to substance use disorder during pregnancy such as thromboembolic disease such as pregnancies complicated
methamphetamine, and perinatal factors such as exposure by diabetes mellitus. (12)(14)
to corticosteroids, as well as preterm birth, low birthweight,
and intrauterine growth restriction. (6) Postnatally, neonates Congenital and Acquired Renal Parenchymal Disease
with increased severity of illness may undergo procedures Congenital anomalies of the kidney and urinary tract can
such as UAC insertions and extracorporeal membrane oxy- cause neonatal hypertension by obstructing the urinary
genation (ECMO), which further increase the risk for hyper- tract. Large cystic kidneys can compress the urinary tract
tension. (10)(11) Additional infant risk factors for hypertension from the outside, causing obstruction at any level, whereas
include renal artery stenosis, renal parenchymal disease, BPD, obstructions at the ureteropelvic junction and the urethra
CoA, endocrine abnormalities, prematurity, and growth re- are more common from valves or malformations within
striction. (1)(2)(14) the urinary tract itself. (14)(20) Polycystic kidney disease,
multicystic dysplastic kidney disease, and congenital renal
hypoplasia/dysplasia likely cause hypertension because of
ETIOLOGY
the associated abnormal renal parenchyma. (12)(20)(21)
The most common cause of neonatal hypertension is renal Postnatal renal ultrasonography can confirm these struc-
vascular disease or UAC-associated thrombosis (12)(14)(16) tural abnormalities, though ideally, these conditions are
followed by congenital or acquired renal parenchymal con- known or suspected antenatally. (10)
ditions. (1)(10)(12)(15)(17)(18) BPD is the most common Acute kidney injury (AKI) is a common yet underdiag-
nonrenal cause of hypertension in infants. The most com- nosed entity in the NICU that results in acquired renal
mon cardiac cause of hypertension in neonates is CoA. parenchymal diseases such as acute tubular necrosis, in-
Postoperative hypertension can occur in infants with in- terstitial nephritis, or cortical necrosis, and subsequent
creased thoracoabdominal pressure after abdominal wall hypertension from excessive renin release. (12)(14)(20)
closure or ECMO. Broad neonatal conditions associated with
hypertension include neurologic causes, such as seizures Bronchopulmonary Dysplasia-related Hypertension
or intracranial masses, and endocrine diagnoses, such BPD is the most common nonrenal cause of hypertension
as hyperthyroidism, mineralocorticoid excess, congenital in infants. Hypertension in BPD may be from a combination
adrenal hyperplasia, or genetic etiologies. (12) Despite this of underlying disease and its treatments. Neonates with BPD
vast number of etiologies, half of neonatal hypertension is are more likely to have vasomotor dysfunction and increased
idiopathic. (19) stiffness of the systemic arteries leading to hypertension.
Vol. 25 No. 1 JANUARY 2024 e39
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
(14)(20) This systemic hypertension is likely how neonates Postoperative Hypertension (Pain, Withdrawal, Fluid
with BPD maintain pulmonary blood supply in the setting of Shifts, Compression, Indirect Endocrine Effects)
concurrent pulmonary hypertension and chronic hypoxemia. Agitation due to undertreated pain or sedation withdrawal
In addition, a vast majority of infants with BPD-associ- may cause hypertension. Postprocedural fluid shifts in the
ated hypertension were found to have low renin levels, thoracoabdominal cavity during prolonged surgical proce-
suggesting that volume excess is a possible contributor to dures can also cause hypertension, (26) particularly after
their hypertension and lung disease. (12)(14)(22)(23) staged repair of giant omphalocele (27) and ECMO. (28)(29)
Treatments for BPD, including corticosteroids and neph- An additional mechanism of postoperative hypertension
rocalcinosis-inducing diuretics, may further exacerbate is endocrine. After abdominal wall closure, increased intra-
hypertension. (12)(20) abdominal pressure causes renal and adrenal compression, re-
sulting in catecholamine release and subsequent hypertension.
(26)(27) Similar postoperative catecholamine release is com-
Coarctation of the Aorta mon after surgical repair of CoA as well, termed “paradoxical
The most common cardiac cause of hypertension in neo- hypertension.” (26)(30)(31) The endocrine component of post-
nates is CoA. (10) Development of CoA antenatally has ECMO hypertension is more variable, as some fluid overload
been attributed to decreased blood flow through the aortic appears to be secondary to impaired water and sodium regula-
valve and aortic arch causing decreased growth (24) and tion without evidence of abnormal renin levels, (26) whereas
postnatally has been attributed to overgrowth of ductal tis- others have increased renin and endothelin levels along with
sue into the aorta causing constriction at those sites. (25) oliguria. (28)(29)
Normally, blood pressure is 10% to 20% higher in the
legs than in the arms. (10) However, in infants with CoA, Endocrine-related Hypertension
increased pressure is required in the aorta proximal to the Hyperthyroidism and congenital adrenal hyperplasia are
area of coarctation to get blood past the narrowed segment common endocrine etiologies for hypertension, especially
and to perfuse the lower body. (25) This increased after- in the context of abnormal physical examination findings
load typically manifests as upper extremity hypertension (Table 3) or elevated thyroid hormone or serum androgen
relative to the lower extremities. Left ventricular hypertro- levels. (10)(32)(33) Endogenous cortisol surges or stress-
phy and the development of collateral vessels are addi- dose steroids for adrenally insufficient neonates likely con-
tional adaptive mechanisms that develop over time in tribute to episodic hypertension as well. Similar surges of
response to obstruction in CoA physiology. (24)(25) catecholamines and other vasoactive hormones can occur
Table 3 . Pertinent Physical Examination Findings
System Finding Possible Etiology
Vital signs Tachycardia Hyperthyroidism
Irregular respiratory rate Intracranial hypertension
Height, weight Poor growth with low percentiles Chronic renal failure
HEENT Elfin facies Williams syndrome
Webbed neck Turner syndrome
Moon facies Cushing syndrome
Proptosis, thyromegaly, goiter Hyperthyroidism
Chest Murmur, thrill, apical heave Structural heart disease, left ventricular hypertrophy
Widely spaced nipples Turner syndrome
Abdomen Abdominal mass Wilms tumor, Neuroblastoma
Palpable kidneys Hydronephrosis
Genitourinary Ambiguous or virilized genitalia Congenital adrenal hyperplasia
Neurologic Anterior fontanelle pulsation Severe intracranial hypertension, Vein of Galen
Skin Pallor, flushing, diaphoresis Pheochromocytoma
Caf!e-au-lait spots Neurofibromatosis
Adenoma sebaceum Tuberous sclerosis
Malar rash Neonatal lupus erythematosus
These findings suggest an etiology beyond the common hypertensive phenotypes in the NICU. (10) HEENT5head, ears, eyes, nose, and
throat examination.
Adapted from Flynn JT, Kaelber DC, Baker-Smith CM, et al; Subcommittee on Screening and Management of High Blood Pressure in Chil-
dren. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;
140(3):e20171904. (10)
e40 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
as a result of compression from intra-abdominal masses, identified as a potential contributor to neonatal hyperten-
tumors (ie, neuroblastoma, Wilms tumor), and operations, sion through increased activation of the mineralocorti-
as noted above. (26) coid receptors, although more research is needed to further
define this purported effect. (19)(39)(40)
Prematurity or Growth Restriction
Preterm and/or growth-restricted small for gestational age PRESENTATION
(SGA) neonates are at an increased risk for hypertension Hypertension in neonates is generally asymptomatic and
because of renal immaturity and vascular maldevelopment. is usually an incidental observation on routine monitoring.
When an infant is born early, in utero renal development is However, there are specific clinical scenarios that have addi-
interrupted, resulting in reduced numbers of nephrons and tional elements to the presentation of neonatal hypertension.
glomeruli, making these neonates particularly vulnerable These common conditions can often be identified by patient
to postnatal injuries from hypoxia, hypotension, or nephro- examination and history review.
toxins. (14) Preterm birth also interrupts normal vasculo-
genesis, causing decreased elastin production. (16)(34)(35) UAC/Vascular and Renal Parenchymal Factors
Similarly, a fetus with growth restriction secondary to preg- Presentation of renal-related hypertension in the NICU
nancy complications, such as malnutrition or hypertension, can occur at any time. When occurring in the first 2 weeks
may require increased circulatory pressure to maintain per- after birth, in situ UAC (20)(41) and severe bilateral con-
fusion in the setting of high in-utero afterload. (14)(17) genital renal parenchymal diseases such as autosomal re-
Persistence of this hypertensive state after birth, despite res- cessive polycystic kidney disease (21) are the most likely
olution of the antenatal environment, highlights the de- suspects. Unilateral renal conditions and thromboembo-
creased arterial compliance that develops with growth lism may be seen in neonates after this period, because
restriction. When these infants undergo subsequent ac- symptoms take time to manifest. (21) Severe refractory hy-
celerated catch-up growth, increased fluid and sodium pertension in a neonate that requires multiple antihyperten-
demands worsen their hypertension. (14)(18) Decreased sive agents suggests congenital conditions such as polycystic
serum insulinlike growth factor 1 levels in SGA infants kidney disease or hormone-producing tumors if hyperten-
have been shown to affect central aortic elastic proper- sion occurs with concurrent hypercalcemia. Physical exami-
ties and structure, resulting in decreased compliance nation finding of the abdominal bruit is specific for renal
and hypertension. (36) artery stenosis, whereas the rare but specific triad of gross
Low-birthweight infants are prone to develop hyperten- hematuria, thrombocytopenia, and palpable flank mass sug-
sion after exposure to steroids because of increased gluco- gests renal vein thrombosis.
corticoid sensitivity, a mechanism that may be mediated
by placental 11b-hydroxysteroid dehydrogenase or endothe- BPD-associated Hypertension
lial nitric oxide synthase. (6)(17) Antenatal glucocorticoid BPD-associated hypertension typically presents later dur-
exposure, particularly within 7 days of birth, results in ing the NICU hospitalization, ranging from 40 weeks’
decreased glomerular filtration and increased levels of PMA to a median of 4 months’ postnatal age. (14)(23)
angiotensin-converting enzyme (ACE) and angiotensin-II, While there is a known association between BPD and risk
whereas postnatal exposure manifests as myocardial dys- of hypertension, (42) presentation can be as subtle as a
function and systemic hypertension from renin-angiotensin- 5 mm Hg increase in blood pressure compared to age-
aldosterone system dysregulation. (17)(35) matched infants, (43) without any correlation between
There can be a few iatrogenic reasons for neonatal hyper- the severity of BPD and severity of hypertension. In addi-
tension in the setting of prematurity. Preterm infants often tion, hypertension resolves independently of the lung dis-
require parenteral nutrition, placing them at risk of receiving ease, with resolution by 12 months’ postnatal age in most
excessive water, sodium, calcium, and vitamin A/D. (14)(18) cases. (14)(23)
Medications such as caffeine and phenylephrine or atropine
mydriatic ophthalmic drops further increase the risk of hy- Coarctation of the Aorta
pertension in this cohort. (37) Environmental phthalate Typically, neonatal coarctation presents with upper ex-
exposure from noninvasive respiratory support equip- tremity blood pressures 10 to 20 mm Hg higher than
ment, (22)(38) parenteral nutrition storage bags, (18) and lower extremity blood pressures, with larger gradients con-
intravenous (IV) line tubing (18)(22) has recently been cerning for longer segments of narrowing. (10)(24) The
Vol. 25 No. 1 JANUARY 2024 e41
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
decreased amount of blood perfusing the lower extremities calm, sleeping versus agitated because of pain or hunger
can also present as a brachial-femoral pulse delay. (25) versus discomfort because of opioid withdrawal symptoms,
However, depending on the location of coarctation, edema, work of breathing)
neonates with CoA can also present with no blood pres- • Laboratory studies:
sure gradient if they have a patent ductus arteriosus 8 Serum laboratories: Electrolyte panel, including
(PDA) and no obstruction at the time of blood pressure calcium, urea, and creatinine to evaluate for kid-
measurement (Table 4). This scenario results in normal ney disease
pulse oximetry values and a passed critical congenital heart 8 Urine studies: Urinalysis for hematuria or proteinuria;
disease screen in the nursery or during short NICU stays. urine calcium and creatinine measurement; and urine
(10)(24)(25) In these patients, symptoms may develop after culture if there are anatomic risk factors or a history
hospital discharge or may remain undetected at home as of urinary tract infection
the ductus arteriosus constricts and closes, creating critical • Imaging:
CoA. (44) Because the left ventricle does not have time to 8 Echocardiography to evaluate for structural heart dis-
adapt to the increased afterload by hypertrophying and gen- ease, CoA, left ventricular hypertrophy, left ventricle
erating collateral circulation, these neonates may present in systolic dysfunction, and left atrium dilation
shock, with florid signs of heart failure including hepato- 8 Renal ultrasonography to evaluate for parenchymal or
megaly, oliguria, lethargy, pulmonary edema, hypotension, structural abnormalities with Doppler of renal arteries
and cardiovascular collapse. (14)(25) If a CoA has been sur- and renal veins; ultrasonography of abdominal aorta if
gically repaired and the infant develops new or worsening there is a history of UAC
upper extremity hypertension, re-coarctation should be sus-
Consultations to consider are as follows:
pected. (10)(44)
• Pediatric nephrology
• Pediatric cardiology for the evaluation of cardiac struc-
DIAGNOSTIC EVALUATION
ture and function
Once hypertension has been identified, diagnostic evalua- • Pediatric interventionalist or pediatric cardiac surgeon if
tion includes the following (10)(12)(20)(22)(25)(37): concern for CoA or renovascular thromboembolism, al-
• Pertinent antepartum history and neonatal history, in- though the degree of intervention may be limited by
cluding a history of postnatal invasive procedures size
• Four-extremity blood pressure, with the infant in a calm • Pediatric ophthalmology if concerns for hypertensive ret-
state inopathy
• Complete physical examination with attention to find- Other studies to consider are as follows:
ings that suggest an uncommon etiology (Table 3) or • Cranial ultrasonography to evaluate for intracranial hem-
end-organ damage to the kidneys, heart, eyes, and orrhage, mass, and cerebral edema
central nervous system (ie, brachial-femoral pulse de- • Thyroid function tests
lay, AKI, left ventricular hypertrophy, hypertensive ret- • Cortisol level
inopathy, encephalopathy, altered mental status, or • Renin and aldosterone levels
seizures) • Adrenal steroid hormone levels to determine specific en-
• Evaluation of medications (corticosteroids, anticholinergic zyme deficiency (particularly 17-hydroxyprogesterone if
eye drops, vasoactive infusions, parenteral nutrition con- there is high suspicion for congenital adrenal hyperpla-
tents, opioid wean) and relative infant state (comfortable, sia secondary to 21-hydroxylase deficiency)
Table 4 . Instances When CoA Does Not Present with Blood Pressure Differential. (10)(24)(25)
Four-Extremity Blood Pressures Likely Anatomy
No gradient Left or right aortic arch with PDA and CoA (10)(24)
No gradient between the right arm and the right leg (10) or Left aortic arch and aberrant origin of the right subclavian artery
higher blood pressure in the left arm than the right arm (25) distal to the CoA (10)(25)
No gradient between the right arm and the right leg (10) Right aortic arch with stenosis in the transverse arch proximal to
the right subclavian artery (10)
No gradient between the left arm and the left leg (10) Left subclavian artery is hypoplastic or stenotic or arises distal to
the CoA (10)(25)
CoA5coarctation of the aorta, PDA5patent ductus arteriosus.
e42 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
• Karyotype to determine genetic sex clinical status, and evidence of end-organ damage. (2)(12)
• Results of the newborn metabolic screen, specifically for Experts recommend that an asymptomatic neonate with a
thyroid level, as well as acylcarnitine, free carnitine, and systolic pressure consistently between the 95th and 99th
ornithine, which are metabolites that are associated with percentiles (Table 1 if <2 weeks old; Table 2 if >2 weeks
maternal diabetes mellitus and hypertensive disorders that old) and with no end-organ involvement may be observed
affect the neonate (45) and does not require treatment because of expected resolution
• Urinary catecholamines if suspicion for pheochromocy- over time in most cases. (1)(12)(46) However, if blood pres-
toma or neuroblastoma sure continues to remain above the 99th percentile despite
• Urinary phthalate levels addressing correctable causes (2)(6)(12)(14) or if there is evi-
dence of end-organ damage at any blood pressure percentile,
GENERAL MANAGEMENT APPROACH experts advocate antihypertensive treatment to prevent adverse
Due to small-scale studies and a lack of evidence on the effects. (1)(9)
long-term effects of hypertension and on the safety and ef- The optimal time course over which blood pressure
ficacy of antihypertensive medications, guidelines for the should be reduced is also not well-established for neonates
treatment of hypertension in neonates and infants are because of limited data on the treatment of severe hyper-
lacking. (36) As such, the treatment of neonatal hypertension tension in neonates. Extrapolating from pediatric guide-
relies on expert consensus and research pertaining to the lines intended for the ambulatory setting suggests blood
treatment of childhood, adolescent, and adult hypertension, pressure should be treated until it is below the 90th per-
which does not always translate well to neonates. (12) centile to minimize end-organ damage. (12) However, the
Nonpharmacologic treatment begins by assessing modifi- approach from a recent pediatric case series (January 2023)
able factors such as fluids, medications, and pain. Volume- (46) may be more applicable to infants in intensive care
overload states may be treated by decreasing IV fluids, and units, although it has not been studied in neonates. This
high-afterload states may be treated by decreasing the doses of case series on previously normotensive patients (age 1
inotropes, vasopressors, and calcium infusions. Other states of month to 18 years) with hypertensive emergency admitted
discomfort that contribute to elevated blood pressure such as to the pediatric intensive care unit suggested a controlled
opioid withdrawal or inadequately controlled pain can be reduction of blood pressure to the 95th percentile over the
treated with analgesic medications. (1)(2)(16) course of 2 days to account for a delayed return of cerebral
Given the lack of clear guidelines regarding pharmaco- autoregulation. (46) The rationale behind this approach is
logic treatment, experts suggest the definitions and treat- applicable to preterm neonates who are at risk of intracra-
ment thresholds shown in Table 5. (9)(12) Generally, the nial hemorrhage because of fragile periventricular vessels
decision to initiate antihypertensive treatment focuses on and was successfully executed in 28 preterm and term infants,
the following 3 factors: blood pressure percentile for PMA, as described in the case series. (12)(37)(46)(47) Additional
Table 5 . Clinical Definitions and Treatment Thresholds
BP Percentile for End-Organ
Stage PMA Clinical Status Involvementa Treatment Type of Treatment
Normal <95th percentile – – No –
Mild hypertension 95th–99th percentile Healthy No No, observe –
Hospitalized or CKD No Consider treatment Oral or IV
Moderate 95th–99th percentile Healthy Yes Treat Oral
hypertension Hospitalized or CKD Yes Treat Oral or IV
Severe >99th percentile Healthy No Treat Oral
hypertension Healthy, hospitalized, Yes Treat IV
or CKD
Hypertensive SBP >120 or DBP Healthy, hospitalized, – Treat IV infusion
emergency >90 (term) or or CKD
hypertension DBP >80
(preterm)
The definitions and treatment thresholds were proposed in 2019 by Harer and Kent (12) based on data from the Dionne et al (9) study on
infants older than 2 weeks.
BP5blood pressure, CKD5chronic kidney disease, DBP5diastolic blood pressure, IV5intravenous, PMA5postmenstrual age, SBP5systolic
blood pressure.
a
End-organ involvement: left ventricular hypertrophy, altered mental status, and acute kidney injury.
Reprinted with permission from Kiss et al. (4).
Vol. 25 No. 1 JANUARY 2024 e43
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
considerations for neonates include the relative immaturity of • Esmolol is a very short-acting IV cardioselective b-blocker
neonatal kidneys and AKI, warranting more caution when us- that is ideal for the treatment of hypertensive infants with
ing antihypertensives that are cleared by the kidneys. (14)(16) CoA. (26)
Consultation with a pediatric nephrologist with experience in • b-blockers should generally be avoided in infants with
this population may be helpful in this situation. (12) BPD because of the risk of bronchoconstriction. (14)
ANTIHYPERTENSIVE AGENTS Calcium Channel Blockers
• Amlodipine and isradipine are enteral calcium channel
Common pharmacotherapeutic interventions include thia-
blockers. Both medications are easy to administer to in-
zide and loop diuretics, ACE inhibitors, calcium channel
fants in compounded solution form and do not require
blockers, b-blockers, and vasodilators. (37) Generally, IV
intensive electrolyte monitoring. (14)
agents should be used if there are signs of end-organ dam-
• Due to its shorter duration of action, isradipine can be
age, and continuous infusions are recommended for the
initiated for BPD-associated hypertension, with a transi-
ability to quickly titrate dosing and closely control blood
tion to amlodipine once blood pressure is controlled. (14)
pressure swings. (28) When initiating and titrating IV an-
• Nicardipine has a short half-life and causes peripheral
tihypertensive therapy, care should be taken to reduce
vasodilation. It is given as a continuous IV infusion and
blood pressure slowly to avoid cerebral ischemia and hem-
is particularly useful for infants on ECMO with severe
orrhage. (48) If the infant is able to tolerate enteral medi-
hypertension. (28)
cations in the absence of severe hypertension, oral/enteral
• Of note, in situations with acutely depressed myocardial
antihypertensive agents can be considered. Agents with a
contractility, caution is advised with calcium channel
long half-life and slower onset of action are most appropri-
blockers because calcium influx is a key component of
ate for hemodynamically stable neonates. (12)(16)
the cellular action potential.
Diuretics Vasodilators
• Diuretic therapy helps treat hypertension and improves • Hydralazine is a vasodilator that can be used for inter-
pulmonary function in infants with BPD. (14) mittent IV treatment of a patient who cannot tolerate en-
• Thiazide diuretics (such as chlorothiazide and hydrochloro- teral medications; however, it may lead to an abrupt
thiazide) are commonly used in the NICU because of ease decrease in blood pressure. (12)
of administration and less frequent electrolyte disturbances • Sodium nitroprusside is also an IV infusion that can be use-
(37) as compared to loop diuretics (ie, furosemide) that can ful for infants on ECMO with severe hypertension refractory
lead to an increased risk of electrolyte derangements (ie, to nicardipine, although there is a risk of toxic metabolite
hyponatremia, hypochloremia, metabolic alkalosis, hypocal- accumulation (cyanide, thiocyanate, and methemoglobin)
cemia, hypomagnesemia) and nephrocalcinosis because of (47), especially in cases of decreased kidney or liver func-
hypercalciuria. (12) tion. (48)
• The aldosterone receptor antagonist spironolactone may
have an adjunct role in the treatment of BPD-associated
ACE Inhibitors/Angiotensin Receptor Blockers
hypertension because of its potassium-sparing mecha-
• This class of antihypertensives is contraindicated in pa-
nism; however, it has a weak diuretic activity. (14)(19)(23)
tients with bilateral renovascular disease or with solitary
kidney and is generally not recommended for infants
b-Blockers less than 44 weeks’ PMA because of potential adverse
• Propranolol is a nonselective b-1 and b-2 blocker that effects on renal development. (11)(20)(48)
may be given orally for the treatment of hypertension; a • ACE inhibitors and angiotensin receptor blockers can
rare adverse effect of propranolol is bradycardia. cause hyperkalemia, AKI, and hypotension; these agents
• Labetalol is a combined b and a-1 blocker with a rapid should be used with extreme caution. When using ACE
onset of action. It is given intravenously and can be inhibitors in preterm infants, consultation with pediatric ne-
used to treat acute hypertension in a patient who cannot phrology is recommended for assistance in determining the
have enteral medications. It is useful because it does not starting dose because of known exaggerated/prolonged hy-
cause tachycardia. (12) potensive response. (12)(26)
e44 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
• In specific situations of older infants with systemic hy- more likely to develop hypertension after NICU discharge,
pertension and concurrent impaired left ventricular dia- at 22 to 26 months of age, (14)(51) and may be at increased
stolic function, enalapril may have a role in improving risk of developing chronic kidney disease. (52) This under-
ventricular dysfunction, as shown in a small observa- scores the importance of diagnosing hypertension in the
tional case series of 11 infants who had resolution of hy- NICU so that appropriate follow-up screening and care can
pertension to the 95th percentile and improved left be provided.
ventricular diastolic dysfunction on serial echocardio- Nephrology follow-up, in particular, is recommended for
grams after 2 weeks of enalapril treatment. (35) infants with congenital renal parenchymal disease, obvious
• Captopril has been studied in neonates, with a starting renal anomalies, and some forms of renovascular hyperten-
dose one-tenth of the normal pediatric starting dose, sion such as renal artery stenosis and renal vein thrombosis
and close monitoring for hyperkalemia and AKI. (48) because of the likelihood of persistent hypertension requir-
ing potentially prolonged treatment with antihypertensives
PROGNOSIS, FOLLOW-UP, AND LONG-TERM or interventions such as stenting. (2)(10)(12)
MONITORING
Although more than 50% of neonates in the NICU with
BPD-related Hypertension
hypertension require antihypertensive medications, (13)(15)
Most BPD-associated hypertension resolves by 2 years of age,
most neonatal hypertension resolves by 1 to 2 years of age.
and antihypertensives can be discontinued by 8 to 12 months
(1)(16)(20) Treatment duration most commonly ranges
of age. (2)(12) A small number may have persistent hyperten-
from 10 days to 8 to 12 months, (12)(14)(15) with almost all
sion, increasing risk of chronic kidney disease later in life.
patients normotensive and off pharmacologic therapy by
(14)(23) In addition, up to half of infants with BPD-associated
24 months’ postnatal age. (20)
hypertension may first present with elevated blood pressure
Long-term outcomes of neonatal hypertension are not
fully known. (12) The American Academy of Pediatrics rec- after NICU discharge, highlighting the importance of on-
ommends routine blood pressure monitoring in all children going blood pressure monitoring in infants with BPD.
after NICU discharge because of increased risk of develop- (9)
ing hypertension, cardiovascular complications, and chronic
kidney disease in childhood and beyond. (2)(10)(34)(35) For Coarctation of the Aorta
affected neonates without an etiology for neonatal hyperten- Infants with hypertension related to congenital heart dis-
sion, it is unclear how long the hypertension will persist. ease and abnormal cardiac anatomy are at increased risk
(12) What is clear, however, is the need for follow-up and of developing hypertension that persists into childhood,
ongoing blood pressure monitoring of these patients, as 1% (2) even after repair of the heart disease. (53) This risk
to 5% of all neonates are hypertensive on follow-up after is even higher in those with a history of preterm birth
NICU discharge, with up to 7.3% remaining hypertensive and CoA specifically, despite neonatal surgical repair.
at 3 years of age. (10)(14)(49) (10)(44)(54) The risks of persistent hypertension and re-co-
In addition, neonates with UAC/vascular thrombosis, arctation are why follow-up is recommended by the American
renal parenchymal disease, BPD, CoA, and prematurity or Academy of Pediatrics and the American Heart Association.
SGA, are at risk for hypertension later in life, even if their
(6)(10)(24) If any suspicion for CoA exists in a newborn after
neonatal hypertension resolves. (16) As such, they require
discharge, follow-up with a pediatric provider who will evalu-
ongoing general pediatric follow-up to monitor for the re-
ate pulses, measure 4-extremity blood pressures, and have a
currence of hypertension. (11) Some may require further
low threshold for echocardiographic evaluation is imperative.
subspecialty care to evaluate for complications of their
(24)(25)
neonatal hypertension, such as retinopathy or congestive
heart failure. (37)(50)
Postoperative Hypertension
UAC/Renovascular and Renal Parenchymal Disease Most cases of postoperative hypertension resolve, but in rare
Hypertension from vascular thrombosis or AKI eventually cases, neonates with repaired giant omphaloceles may need
resolves once the inciting agent is removed and the throm- antihypertensive therapy after NICU discharge, requiring
bus has resolved. (1) However, despite the resolution of hy- outpatient follow-up with a provider able to manage and
pertension in the NICU, preterm infants with AKI are wean those medications. (27)(37)
Vol. 25 No. 1 JANUARY 2024 e45
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
Table 6 . Management Considerations for Common Clinical Conditions of Hypertension in the NICU.
Recommended
Follow-up for
Surveillance and
Clinical Context Timing of Specific Aspects of Pharmacologic Management Management of
of Hypertension Presentation Presentation Treatment Considerations Hypertension
UAC or First 2 wks after birth: Abdominal bruit, ACE inhibitors If associated with renal Nephrology for
renovascular UAC gross hematuria, contraindicated artery stenosis or renovascular
2 wks after birth: thrombocytopenia, with renovascular aortic thrombosis, abnormalities
thromboembolism flank mass disease the duration of
Heparin or papaverine therapy is weeks to
(reduces the risk of months
UAC thrombosis) Once normotensive for
(20)(34) 4–8 wks and
thrombus is
resolved,
antihypertensive
medication can be
weaned (1)
Renal First 2 wks after May be known ACE inhibitors Postnatal renal Nephrology
parenchymal, birth: severe antenatally contraindicated ultrasonography
congenital, or bilateral Palpable kidneys with solitary kidney (10)
acquired conditions AKI or history of AKI and until at least Avoid nephrotoxins
2 wks after birth: 44 wks’ PMA Some congenital renal
unilateral conditions may
conditions require multiple
antihypertensive
medications (2)(20)
BPD-associated Later during NICU None or a subtle β-blockers Glucocorticoid-induced Pediatrician
hypertension hospitalization, 40 elevation (as little contraindicated hypertension Pulmonology
wks to 4 months’ as 5 mm Hg Diuretics: thiazide (ie, resolves once
PMA above normal), chlorothiazide) treatment is
Mostly resolves by concurrent BPD, or preferred over loop stopped; consider
12 months’ PMA chronic lung diuretic discontinuing
disease Calcium channel or reducing the
blockers dose (1)
Spironolactone Fluid overload and
(emerging role) hypoxemia can
(11)(19)(23) contribute (11)
Aortic coarctation Any time during the Arch obstruction: Prostaglandin infusion Specific situations Pediatrician
newborn period, " Upper extremity (ductal patency) (Table 5) require Cardiology
whether in- hypertension β-blockers close monitoring Cardiothoracic
hospital or at (10–20 mm Hg until PDA is closed surgery
home gradient) (24)
May not be detected " Brachial-femoral Surgery is definitive
by pulse oximetry pulse delay treatment (10)
screening for " Heart failure (ie, Re-coarctation is
critical congenital pulmonary edema, possible, even after
heart disease hepatomegaly, surgical repair
oliguria, lethargy)
and eventual shock
No obstruction:
no gradient in
extremity blood
pressures, may
have murmur of
PDA
Postoperative After procedures for Episodic, may have Adequate sedation Address pain and Pediatrician if
hypertension abdominal wall signs of fluid and analgesia fluid/volume requiring
closure, overload (ie, (11)(29) overload (29) antihypertensive
omphalocele edema), oliguria, Vasodilators: nicardipine Discontinue or reduce medications (27)
repair, or ECMO or agitation/ (preferred) (28) or the dose of
distress nitroprusside (47)(49) inotropes and
for continuous steroids, if possible
infusions; hydralazine
(13) for intermittent
IV doses
Consider labetalol (13)
Continued
e46 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
Table 6 . (Continued)
Recommended
Follow-up for
Surveillance and
Clinical Context Timing of Specific Aspects of Pharmacologic Management Management of
of Hypertension Presentation Presentation Treatment Considerations Hypertension
Endocrine Any time Clitoromegaly in If strong suspicion of Endocrinology Depends on the
female infants, and endocrinopathy, consultation etiology
signs of consider initiating Consider special Endocrinology
hyperthyroidism or treatment while studies (see the
catecholamine results are pending Diagnostic
excess Evaluation section)
Obtain newborn
screen results
Prematurity or Any time May be known ACE inhibitors Evaluate medications Pediatrician
growth antenatally contraindicated and consider Nephrology
restriction Low birthweight until at least discontinuing or
May suggest 44 wks’ PMA reducing doses of
suboptimal in caffeine,
utero environment phenylephrine, or
(ie, obesity, atropine mydriatic
hypertension, ophthalmic drops
malnutrition) (17) (37)
Avoid nephrotoxins
If receiving parenteral
nutrition, evaluate
constituents (free
water, sodium,
calcium, vitamin A,
vitamin D) (11)(18)
If concurrent
myocardial
dysfunction, may
require multiple
antihypertensive
medications (13)
Medication contraindications are in bold. ACE5angiotensin-converting enzyme, AKI5acute kidney injury, BPD5bronchopulmonary dysplasia,
ECMO5extracorporeal membrane oxygenation, PDA5patent ductus arteriosus, PMA5postmenstrual age, UAC5umbilical artery catheter.
CONCLUSION
the normal range of pressures and pressure
Overall, more information is needed on the causes and con- patterns.
sequences of neonatal hypertension to improve recognition • Know the pathophysiology of common scenarios
of the condition. More robust data are needed on long-term
in the NICU in which a neonate presents with
outcomes of neonatal hypertension to provide optimal treat-
systemic hypertension.
ment, monitoring, and follow-up of this chronic condition.
• Formulate a differential diagnosis for neonatal
(1)(12)(37)(55) Based on limited data, we can use blood pres-
hypertension.
sure reference ranges to diagnose hypertension and expert
guidelines to help with treatment decisions for hyperten- • Know the clinical and diagnostic features of an
sion that are common in the NICU (Table 6). Understand- infant with systemic hypertension, including
ing hypertension in the clinical context of the underlying laboratory and imaging studies.
disease or procedures may help management decisions. • Know the management of an infant with systemic
hypertension, including adverse effects.
American Board of Pediatrics
Neonatal-Perinatal Content
Specifications References
• Know the factors that regulate systemic blood 1. Janjua HS, Batisky DL. Renal vascular disease in the newborn. In:
Gleason CA, Devaskar SU, eds. Avery’s Diseases of the Newborn. 9th
pressure in term and preterm infants and know ed. Philadelphia, PA: Elsevier; 2011:1240–1249
Vol. 25 No. 1 JANUARY 2024 e47
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
2. Vogt BA, Springel T. The kidney and urinary tract of the neonate. 22. Farnbach K, Iragorri S, Al-Uzri A, Rozansky D, Forbush R, Jenkins
In: Martin RJ, Fanaroff AA, Walsh MC, eds. Fanaroff and Martin’s R. The changing spectrum of hypertension in premature infants.
Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant. 11th ed. J Perinatol. 2019;39(11):1528–1534
Philadelphia, PA: Elsevier; 2019:1871–1895 23. Jenkins RD, Aziz JK, Gievers LL, Mooers HM, Fino N, Rozansky
3. Kent AL, Chaudhari T. Determinants of neonatal blood pressure. DJ. Characteristics of hypertension in premature infants with and
Curr Hypertens Rep. 2013;15(5):426–432 without chronic lung disease: a long-term multi-center study. Pediatr
4. Kiss JK, Gajda A, Mari J, Nemeth J, Bereczki C. Oscillometric Nephrol. 2017;32(11):2115–2124
arterial blood pressure in haemodynamically stable neonates in the 24. Geggel RL. Coarctation of the aorta: delay in diagnosis and referral
first 2 weeks of life. Pediatr Nephrol. 2023;38(10):3369–3378 basis from infancy to adulthood. J Pediatr. 2022;242:57–62
5. Zubrow AB, Hulman S, Kushner H, Falkner B; Philadelphia 25. Hoffman JI. The challenge in diagnosing coarctation of the aorta.
Neonatal Blood Pressure Study Group. Determinants of blood Cardiovasc J Afr. 2018;29(4):252–255
pressure in infants admitted to neonatal intensive care units: a 26. Starr MC, Flynn JT. Neonatal hypertension: cases, causes, and
prospective multicenter study. J Perinatol. 1995;15(6):470–479 clinical approach. Pediatr Nephrol. 2019;34(5):787–799
6. Dionne JM. Determinants of blood pressure in neonates and 27. Peranteau WH, Tharakan SJ, Partridge E, et al. Systemic
infants: predictable variability. Hypertension. 2021;77(3):781–787 hypertension in giant omphalocele: an underappreciated association.
7. Kent AL, Kecskes Z, Shadbolt B, Falk MC. Blood pressure in the J Pediatr Surg. 2015;50(9):1477–1480
first year of life in healthy infants born at term. Pediatr Nephrol. 28. Liviskie CJ, DeAvilla KM, Zeller BN, Najaf T, McPherson CC.
2007;22(10):1743–1749 Nicardipine for the treatment of neonatal hypertension during
8. Nist MD, Backes CH, Moorehead P, Wispe J. Blood pressure extracorporeal membrane oxygenation. Pediatr Cardiol.
support in the very low-birth-weight infant during the first week of 2019;40(5):1041–1045
life. Adv Neonatal Care. 2012;12(3):158–163, quiz 164–165 29. Maratta C, Potera RM, van Leeuwen G, Castillo Moya A, Raman
9. Dionne JM, Abitbol CL, Flynn JT. Hypertension in infancy: L, Annich GM. Extracorporeal life support organization (ELSO):
diagnosis, management and outcome. Pediatr Nephrol. 2020 pediatric respiratory ELSO guideline. ASAIO J. 2020;
2012;27(1):17–32 66(9):975–979
10. Flynn JT, Kaelber DC, Baker-Smith CM, et al; Subcommittee on 30. Choy M, Rocchini AP, Beekman RH, et al. Paradoxical hypertension
Screening and Management of High Blood Pressure in Children. after repair of coarctation of the aorta in children: balloon
Clinical practice guideline for screening and management of high angioplasty versus surgical repair. Circulation. 1987;75(6):1186–1191
blood pressure in children and adolescents. Pediatrics. 2017;140(3):
31. Roeleveld PP, Zwijsen EG. Treatment strategies for paradoxical
e20171904
hypertension following surgical correction of coarctation of the aorta
11. Altemose K, Dionne JM. Neonatal hypertension: concerns within in children. World J Pediatr Congenit Heart Surg. 2017;8(3):321–331
and beyond the neonatal intensive care unit. Clin Exp Pediatr.
32. Nimkarn S, New MI. Disorders of the adrenal gland. In: Gleason
2022;65(8):367–376
CA, Devaskar SU, eds. Avery’s Diseases of the Newborn. 9th ed.
12. Harer MW, Kent AL. Neonatal hypertension: an educational review. Philadelphia, PA: Elsevier; 2011:1274–1285
Pediatr Nephrol. 2019;34(6):1009–1018
33. Sorbara JC, Wherrett DK. Disorders of sex development. In: Martin
13. AlMaazmi A, Hagan J, Fernandes CJ, Gowda SH. Neonatal systemic RJ, Fanaroff AA, Walsh MC, eds. Fanaroff and Martin’s Neonatal-
hypertension across the PHIS database: an update. Int J Cardiol. Perinatal Medicine: Diseases of the Fetus and Infant. 11th ed.
2023;376:49–53 Philadelphia, PA: Elsevier; 2019:1665–1705
14. Starr MC, Wilson AC. Systemic hypertension in infants with 34. Bates ML, Levy PT, Nuyt AM, Goss KN, Lewandowski AJ,
bronchopulmonary dysplasia. Curr Hypertens Rep. 2022;24(6): McNamara PJ. Adult cardiovascular health risk and cardiovascular
193–203 phenotypes of prematurity. J Pediatr. 2020;227:17–30
15. Blowey DL, Duda PJ, Stokes P, Hall M. Incidence and treatment of 35. Stanford AH, Reyes M, Rios DR, et al. Safety, feasibility, and impact
hypertension in the neonatal intensive care unit. J Am Soc Hypertens. of enalapril on cardiorespiratory physiology and health in preterm
2011;5(6):478–483 infants with systemic hypertension and left ventricular diastolic
16. Hjorten R, Flynn JT. Neonatal hypertension. Clin Perinatol. dysfunction. J Clin Med. 2021;10(19):4519
2022;49(1):27–42 36. Akazawa Y, Kamiya M, Yamazaki S, et al. Impact of decreased
17. Jebasingh F, Thomas N. Barker hypothesis and hypertension. Front serum insulin-like growth factor-1 levels on central aortic compliance
Public Health. 2022;9:767545 in small-for-gestational-age infants. Neonatology. 2017;111(1):30–36
18. Panneel L, Cleys P, Breugelmans C, et al. Neonatal exposure to 37. Deitrick J, Stevenson K, Nguyen D, Sessions W, Linga V, Vasylyeva
phthalate and alternative plasticizers via parenteral nutrition. Int T. Hypertension secondary to renal hypoplasia presenting as acute
J Pharm. 2023;631:122472 heart failure in a newborn. Clin Hypertens. 2019;25:10
19. Jenkins RD. Phthalates cause a low-renin phenotype commonly 38. Stroustrup A, Bragg JB, Busgang SA, et al. Sources of clinically
found in premature infants with idiopathic neonatal hypertension. significant neonatal intensive care unit phthalate exposure. J Expo
Pediatr Nephrol. 2023;38(6):1717–1724 Sci Environ Epidemiol. 2020;30(1):137–148
20. Watkinson M. Hypertension in the newborn baby. Arch Dis Child 39. Jenkins R, Tackitt S, Gievers L, et al. Phthalate-associated
Fetal Neonatal Ed. 2002;86(2):F78–F81 hypertension in premature infants: a prospective mechanistic cohort
21. Guay-Woodford LM, Desmond RA. Autosomal recessive polycystic study. Pediatr Nephrol. 2019;34(8):1413–1424
kidney disease: the clinical experience in North America. Pediatrics. 40. Jenkins R, Farnbach K, Iragorri S. Elimination of intravenous di-2-
2003;111(5 Pt 1):1072–1080 ethylhexyl phthalate exposure abrogates most neonatal hypertension in
e48 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
premature infants with bronchopulmonary dysplasia. Toxics. 2021; 48. Flynn JT. The hypertensive neonate. Semin Fetal Neonatal Med.
9(4):75 2020;25(5):101138
41. Barrington KJ. Umbilical artery catheters in the newborn: effects of 49. Mhanna MJ, Iqbal AM, Kaelber DC. Weight gain and hypertension
position of the catheter tip. Cochrane Database Syst Rev. at three years of age and older in extremely low birth weight infants.
2000;1999(2):CD000505 J Neonatal Perinatal Med. 2015;8(4):363–369
42. Emery EF, Greenough A. Neonatal blood pressure levels of preterm 50. Goyal P, Agarwal K. Hypertensive retinopathy in a neonate. BMJ
infants who did and did not develop chronic lung disease. Early Case Rep. 2020;13(10):e235720
Hum Dev. 1992;31(2):149–156 51. Askenazi DJ, Heagerty PJ, Schmicker RH, et al; PENUT Trial
43. Alagappan A, Malloy MH. Systemic hypertension in very low-birth Consortium. The impact of erythropoietin on short- and long-
weight infants with bronchopulmonary dysplasia: incidence and risk term kidney-related outcomes in neonates of extremely low
factors. Am J Perinatol. 1998;15(1):3–8 gestational age. Results of a multicenter, double-blind, placebo-
44. Hager A, Kanz S, Kaemmerer H, Schreiber C, Hess J. Coarctation controlled randomized clinical trial. J Pediatr. 2021;232:65–72.e7
Long-term Assessment (COALA): significance of arterial 52. Selewski DT, Charlton JR, Jetton JG, et al. Neonatal acute kidney
hypertension in a cohort of 404 patients up to 27 years after surgical injury. Pediatrics. 2015;136(2):e463–e473
repair of isolated coarctation of the aorta, even in the absence of 53. Greenberg JH, McArthur E, Thiessen-Philbrook H, et al. Long-term
restenosis and prosthetic material. J Thorac Cardiovasc Surg. risk of hypertension after surgical repair of congenital heart disease
2007;134(3):738–745 in children. JAMA Netw Open. 2021;4(4):e215237
45. Reiss JD, Chang AL, Mayo JA, et al. Newborn screen metabolic 54. Di Salvo G, Castaldi B, Baldini L, et al. Masked hypertension in
panels reflect the impact of common disorders of pregnancy. Pediatr young patients after successful aortic coarctation repair: impact on
Res. 2022;92(2):490–497 left ventricular geometry and function. J Hum Hypertens.
46. Coulthard MG. Managing severe hypertension in children. Pediatr 2011;25(12):739–745
Nephrol. 2023;38(10):3229–3239 Doi: 10.1007/s00467-023-05896-z 55. Stewart DL, Barfield WD; Committee on Fetus and Newborn.
47. Holme MR, Sharman T. Sodium nitroprusside. In: StatPearls Updates on an at risk population: late-preterm and early-term
[Internet]. Treasure Island, FL: StatPearls Publishing; 2023 infants. Pediatrics. 2019;144(5):e20192760
Vol. 25 No. 1 JANUARY 2024 e49
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
INDEX OF SUSPICION IN THE NURSERY
A Term Neonate with Encephalopathy
Shruthi Kumar Bharadwaj, MD, DM,* Smriti Bhargava, MD,† Sheila Samanta Mathai, MD, DM,*
Jayashree Purkaystha, MD†
Departments of *Neonatology and †Pediatrics, Kasturba Medical College and Hospital, Manipal Academy of Higher Education, Manipal, Karnataka, India
PRESENTATION
A male infant is born of a nonconsanguineous marriage at 38 weeks of gestation
to a primigravida via normal vaginal delivery at a level 1 hospital. The infant’s
antenatal history is unremarkable, with normal first- and second-trimester scans.
At 30 weeks, a growth scan shows pelvicalyceal system dilation (6 mm) with an
amniotic fluid index of 30. The mother perceives decreased fetal movements the
week before the delivery, but the biophysical profile is normal. Labor is sponta-
neous, and she delivers vaginally after a normal duration of labor. The infant
does not cry at birth and needs resuscitation for 20 minutes without requiring
cardiac compressions. The Apgar score is 5 at 10 minutes after birth, and cord
blood gas is unavailable. He is referred to us (level 3 referral hospital) on nasal
flow oxygen at 3 hours after birth for further management.
On admission, his weight is 2,500 g, and vital signs are stable; however, he has
shallow respiratory efforts. The sensorium is obtunded with reduced spontaneous ac-
tivity, hypotonia, weak cry, poor suck, incomplete Moro reflex, absent gag reflex, and
equal and reactive pupils. He does not have clinical seizures or external dysmorphic
features. Arterial blood gas shows a pH of 7.02, PCO2 of 56 mm Hg (7.45 kPa), bicar-
bonate of 12.2 mEq/L (12.2 mmol/L), base deficit of !14.8 mEq/L (!14.8 mmol/L),
and lactate of 126.7 mg/dL (14.06 mmol/L). A diagnosis of moderate encephalopathy
secondary to perinatal causes is made, and he is started on therapeutic whole body
cooling. Conventional electroencephalogram (EEG), amplitude-integrated EEG, and
neurosonogram are normal. He has evidence of end-organ dysfunction with raised
creatine kinase of 1,887.0 U/L (31.51 mkat/L), troponin T of 0.092 ng/mL (0.09 mg/L),
lactate dehydrogenase of 761 U/L (12.71 mkat/L), aspartate aminotransferase of 67 U/L
(1.12 mkat/L), and activated partial thromboplastin time of 46.1 seconds. Serum creati-
nine is 0.44 mg/dL (38.90 mmol/L), sepsis screen and blood culture are negative, and
the chest radiograph is normal. He remains hemodynamically stable but is electively
placed on mechanical ventilation because of poor sensorium, inadequate breathing ef-
forts, and carbon dioxide retention. Whole body cooling is performed for 72 hours, and
rewarming is completed over 12 hours.
AUTHOR DISCLOSURES Drs Bharadwaj,
Bhargava, Mathai, and Purkaystha have
disclosed no financial relationships DISCUSSION
relevant to this article. This commentary Progression
does not contain a discussion of an
unapproved/investigative use of a The infant’s sensorium improved over the next 3 days, with spontaneous opening
commercial product/device. and normal eye movements, but he remained hypotonic with minimal spontaneous
e50 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
infant’s sensorium improved, the hypotonia persisted de-
spite a normal brain scan and sepsis screen, and the dif-
ferentials were revised, as shown in the Table. (1)(2)(3)
Actual Diagnosis
Whole exome sequencing reveals Centronuclear Myopathy
Type 1 (Autosomal Dominant AD) with Pathogenic strain
for DNM2 (1), VARIANT - c.1102G>A, located at EXON 8.
The Condition
Centronuclear myopathy (CNM) is an inherited congenital
myopathy (CM), which results in muscle weakness, hypoto-
nia, and myopathic features on muscle biopsy. (4) Congenital
myopathies are categorized based on the predominant patho-
logic features as core myopathies (most common), CNM, and
nemaline myopathy. (5)(6) The X-linked form of CNM has a
severe presentation at birth with marked weakness, respira-
tory failure, ophthalmoplegia with hypotonia, and coexisting
birth asphyxia. Autosomal dominant forms present late and
are milder, whereas autosomal recessive forms have an in-be-
tween presentation. (7) Core and nemaline myopathies have
a slow progression. The presence of prominent facial weak-
Video. Neonate with improved sensorium and normal eye movements
with minimal limb movements and hypotonia. ness with feeding problems with or without ptosis, general-
ized hypotonia with hyporeflexia, respiratory and bulbar
limb movements (Video). Although blood gases were normal, muscle weakness, and the absence of tongue fasciculations
he failed attempts at extubation twice because of persistent hint at CNM from other causes in hypotonic infants. (8)
pooling of secretions and the absence of a gag reflex. Given Many of these neonates may succumb in the neonatal period.
normal EEG with persistent hypotonia despite improving sen- The infant in our case presented with generalized hypotonia,
sorium, the possibility of a neuromuscular genetic condition severe respiratory muscle involvement, and evidence of birth
was considered, and whole exome sequencing was ordered. asphyxia. A family history of neonatal deaths or miscarriages
is often present. (9) Polyhydramnios and reduced fetal move-
Differential Diagnosis ments are frequent during pregnancy, as in this case. (10)
Given the need for prolonged resuscitation with encepha-
lopathy, initial differential diagnoses included hypoxic-is- MANAGEMENT
chemic encephalopathy (HIE), intracranial hemorrhage, Electron microscopy of muscle biopsy and genetic testing
early-onset sepsis, and neuromuscular disease. As the aid in diagnosis, prognostication, and counseling. Current
Table. Causes and Presentation of a Hypotonic Infant
Site of Involvement Presentation Example
Central Central hypotonia (truncal hypotonia > peripheral), Hypoxic ischemic encephalopathy, Chromosomal
preserved neonatal reflexes with or without disorders, an inborn error of metabolism,
encephalopathy, seizures structural malformations of the brain
Anterior horn cell Generalized weakness and absent DTRs, preserved Spinomuscular atrophy
sensorium, fasciculations
Nerve Distal muscle weakness and wasting, along with Peripheral neuropathy
decreased DTRs
Neuromuscular junction Involvement of facial muscles with or without Congenital myasthenia gravis, transient
generalized weakness, feeding difficulties myasthenia gravis, botulism
Muscle Generalized weakness, decreased DTRs, respiratory Congenital muscular or myotonic dystrophy,
weakness, preserved sensorium, joint contractures Congenital or metabolic myopathy
DTR5deep tendon reflex.
Vol. 25, No. 1 JANUARY 2024 e51
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
management is supportive and offers multidisciplinary
abnormalities), differential diagnosis, evalua-
team care. Some parents opt for early palliative care in se-
tion, management, and outcomes of neonatal
vere cases. Infants who survive beyond the neonatal period
hypotonia/neuromuscular weakness.
without significant ventilatory requirements need close re-
• Know the causes, clinical features, evaluation,
spiratory function monitoring and sleep studies. Carrier
and management of hypoxic-ischemic
screening enables parents to make informed decisions re-
encephalopathy.
garding subsequent pregnancies.
• Recognize the controversies associated with
the introduction of new genetic tests for rare
PATIENT COURSE
and common diseases that present in the neo-
The infant remained on a ventilator along with enteral tube natal period.
feeds for 22 days. His parents opted for palliative care with
the de-escalation of intensive care. Single-gene sequencing
of both parents was negative, suggesting a de novo or spo-
References
radic mutation, which is known to have a severe presenta-
1. Hill A. Neonatal hypotonia. In: Maria BL, editor. Current
tion. The parents were counseled and offered follow-up
Management in Child Neurology. 3rd ed. Hamilton: B C Decker Inc;
services from the departments of neonatology and medical 2005;529–534
genetics. However, the infant died soon after.
2. Ahmed MI, Iqbal M, Hussain N. A structured approach to the
assessment of a floppy neonate. J Pediatr Neurosci. 2016;11(1):2–6
Lessons for the Clinician 3. Prasad AN, Prasad C. The floppy infant: contribution of genetic and
• HIE often masks an underlying neuromuscular condi- metabolic disorders. Brain Dev. 2003;25(7):457–476
tion; hence, a deeper understanding and strong index of 4. Butterfield RJ. Congenital muscular dystrophy and congenital
suspicion are required for timely diagnosis. myopathy. Continuum (Minneap Minn). 2019;25(6):
1640–1661
• In diagnostic dilemmas, the most commonly occurring
5. Ravenscroft G, Bryson-Richardson RJ, Nowak KJ, Laing NG. Recent
conditions should be considered first and treated until a
advances in understanding congenital myopathies. F1000 Res.
definitive diagnosis is established. In this case, treatment 2018;7:1921
for HIE was given. 6. Jungbluth H, Treves S, Zorzato F, et al. Congenital myopathies:
• With affordable and accessible genetic testing, such chal- disorders of excitation-contraction coupling and muscle contraction.
lenging cases should be considered for further genetic Nat Rev Neurol. 2018;14(3):151–167
evaluation as it is less intrusive. 7. Jungbluth H, Wallgren-Pettersson C, Laporte J. Centronuclear
(myotubular) myopathy. Orphanet J Rare Dis. 2008;3(1):26
8. North KN, Wang CH, Clarke N, et al; International Standard of
American Board of Pediatrics Care Committee for Congenital Myopathies. Approach to the
diagnosis of congenital myopathies. Neuromuscul Disord. 2014;
Neonatal-Perinatal Content 24(2):97–116
Specifications 9. Gilbreath HR, Castro D, Iannaccone ST. Congenital myopathies and
muscular dystrophies. Neurol Clin. 2014;32(3):689–703, viii
• Know the basis for (including genetic), clinical
10. Beecroft SJ, Lombard M, Mowat D, et al. Genetics of
and laboratory features (including associated neuromuscular fetal akinesia in the genomics era. J Med Genet.
2018;55(8):505–514
e52 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
INDEX OF SUSPICION IN THE NURSERY
A Newborn with Inspiratory Stridor
Ishica B. Zaman,* Dana Mazuru-Witten, MD,† Akshaya J. Vachharajani, MD‡
*University of Missouri School of Medicine, Columbia, MO
†
Departmentof Diagnostic Radiology and Pediatrics, University of Missouri, Columbia, MO
‡
Department of Neonatal-Perinatal Medicine and Pediatrics, University of Missouri, Columbia, MO
PRESENTATION
A newborn male infant is born via cesarean delivery at 31 weeks and 2 days’ ges-
tation to a 21-year-old now gravida 1, para 1 woman. Prenatal laboratory results
show that the woman is positive for Rhesus factor, negative for Coombs sign,
negative for human immunodeficiency virus, negative for hepatitis B surface
antigen, negative for syphilis antibody, nonreactive to the rapid plasma reagin,
Rubella immune, and negative for group B Streptococcus. The pregnancy was
complicated by marginal cord insertion, breech presentation, and a family his-
tory of maple syrup urine disease. The neonate undergoes routine resuscita-
tion, and his Apgar scores are 4 and 8 at 1 and 5 minutes, respectively. The
patient is admitted to NICU for prematurity and respiratory distress. The in-
fant is treated with noninvasive positive pressure ventilation (NIPPV) for respi-
ratory distress with a fraction of inspired oxygen (FiO2) of 0.21. At 5 days of
age, he is switched from NIPPV to continuous positive airway pressure with
an FiO2 requirement of 0.21 to 0.23. He continues to require a 4 L/min high-
flow nasal cannula at a postmenstrual age of 36 weeks, establishing the diagnosis of
grade 2 bronchopulmonary dysplasia. He is observed to have inspiratory stridor at a
corrected gestational age of 37 weeks and continues to need respiratory support.
A flexible fiberoptic laryngoscopy is performed by pediatric otolaryngologists,
which reveals a midline cystic structure present at the base of the tongue (Fig 1).
Further evaluation with magnetic resonance imaging shows a 1-cm cystic lesion
arising from the midline base of the tongue (Fig 2).
DIFFERENTIAL DIAGNOSIS
Inspiratory stridor in neonates is most often due to laryngomalacia, but the pres-
ence of a cyst at the base of the tongue expands the differential diagnosis:
• Laryngomalacia
• Thyroglossal duct cyst
AUTHOR DISCLOSURES Drs Zaman,
• Vallecular cyst
Mazuru-Witten, and Vachharajani have
disclosed no financial relationships
PROGRESSION relevant to this article. This commentary
does not contain a discussion of an
Ultrasonography reveals a thyroid gland normal in appearance and anatomic lo- unapproved/investigative use of a
cation and confirms the presence of a cyst. Endoscopic marsupialization of the commercial product/device.
Vol. 25 No. 1 JANUARY 2024 e53
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
Figure 1. Vallecular cyst on flexible fiberoptic laryngoscopy. Figure 2. Cystic structure at the base of the tongue on magnetic reso-
nance imaging.
cyst is performed under general anesthesia, which reveals
with postextubation stridor will get reintubated; therefore,
a vallecular cyst. The patient receives a 7-day course of
there are several tests to ensure airway patency before extu-
amoxicillin and clavulanic acid. No complications develop
bation, such as ultrasonography, video laryngoscopy, and
in the postoperative period, and the patient’s stridor re-
solves. He is weaned off the respiratory support and is dis- the cuff leak test (deflating the cuff of the endotracheal
charged from the hospital after he tolerates oral feeding. tube and covering the proximal opening to listen for any
audible air leaking around the cuff as the patient spontane-
DISCUSSION ously breathes; hearing an air leak would be a positive test
and indicates that there is no laryngeal edema and that it is
Newborns can often present with stridor within a few days
safe to extubate the patient).
after birth, typically because of an acquired or congenital
The vallecula is a small depression in the epiglottic mu-
anomaly of the airway at the laryngeal or tracheobronchial
cosa located at the base of the tongue between the medial
levels. (1) The different causes of stridor can be distin-
and lateral glossoepiglottic folds. It can be found at the
guished by the varying timing of the stridor. Pathologies
transition point between the pharynx and the larynx. Sac-
such as laryngomalacia, vocal cord palsy, and postextuba-
like pockets of tissue containing fluid or air, also known
tion laryngeal edema are common causes of inspiratory
stridor. The expiratory stridor is more often caused by tra- as cysts, can form in the vallecula. These cysts are often
cheal conditions, and biphasic stridor is secondary to glot- the result of an obstructed mucosal duct or can form be-
tic or subglottic pathologies. (1) cause of malformations of the third branchial arch. Vallec-
Laryngomalacia is the most common cause of inspira- ular cysts are a very atypical cause of inspiratory stridor in
tory stridor. (1) The exact etiology is unknown. Diagnosis a newborn. Only 10.5% to 20.1% of all congenital laryn-
is made via laryngoscopy, which reveals collapse of supra- geal cysts are represented by vallecular cysts, making
glottic tissues. Vocal cord paralysis is another potential them quite rare. (3) The overall incidence of vallecular
cause of inspiratory stridor in a neonate. Potential etiolo- cysts is !3.49 to 5.3 cases per 100,000 newborns. (3)
gies include birth-related trauma, iatrogenic injuries, Findings on physical examination can include inspiratory
central nervous system disorders, and idiopathic causes. stridor, respiratory distress, poor feeding, failure to thrive,
Vocal cord paralysis is often associated with dysphagia and snoring, choking, chronic cough, hoarse cry, cyanosis, and
hoarseness, which may help distinguish it from other croup. (4) Larger lesions may lead to life-threatening air-
causes of inspiratory stridor. Diagnosis is made by directly way obstructions.
visualizing the vocal cords via laryngoscopy. (2) Neonates Imaging is important in determining the diagnosis. Flexible
who are intubated can develop laryngeal edema, and two- laryngoscopy is often the initial step in screening infants with
thirds of these patients will present with postextubation stri- upper airway obstruction. The laryngoscope is able to deter-
dor. The endotracheal tube can cause superficial mucosal mine the size and location of the cyst. When the wall of the
inflammation and vocal cord dysfunction, even if only in cyst is thick, it is more difficult to identify whether the lesion
place for a short period. Half of the patients who present is solid or cystic in nature via laryngoscopy. Ultrasonography is
e54 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
the preferred imaging modality over computed tomography be- • A combination of laryngoscopy and ultrasonography is
cause of the risks of radiation with the latter. Ultrasonography used as the main diagnostic tool in the identification of
is able to determine the location and size of the lesion, as well a vallecular cyst. (6)
as the echo and the blood flow to determine whether it is cys- • The treatment of choice for vallecular cysts is removal
tic or solid. Ultrasonography is also useful in observing the via endoscopic marsupialization. (5)
thyroid anatomy. If the thyroid is absent and the lesion is solid,
a diagnosis of ectopic thyroid can be considered and a vallecu-
lar cyst can be ruled down. (5) A combination of laryngoscopy
American Board of Pediatrics
and ultrasonography are used as the main diagnostic tools in
Neonatal-Perinatal Content
the identification of a vallecular cyst. (6) Specifications
Needle aspiration of vallecular cysts is no longer ac- • Know the clinical manifestations and ap-
cepted as adequate treatment because of the high incidence proaches to therapy of neck masses in the
of recurrence. Surgical excision is now the gold standard newborn infant.
for treatment. The 2 main procedures for vallecular cyst re- • Know the various causes of stridor in the new-
moval are endoscopic complete excision and endoscopic born and how to assess severity.
marsupialization. (5) Marsupialization is typically the treat-
ment of choice as it has a low recurrence rate and its safety
and effectiveness are well balanced. (6) Marsupialization References
can be performed using electrocautery, microdissection, 1. Bhatt J, Prager JD. Neonatal stridor: diagnosis and management. Clin
carbon dioxide laser, potassium-titanyl-phosphate laser, or Perinatol. 2018;45(4):817–831
controlled ablation. (5) 2. Ryan MA, Upchurch PA, Senekki-Florent P. Neonatal vocal fold
paralysis. NeoReviews. 2020;21(5):e308–e322
3. Alnaimi A, Abushahin A. Vallecular cyst: reminder of a rare cause of
stridor and failure to thrive in infants. Cureus. 2021;13(11):e19692
Lessons for the Clinician 4. Al-Mahboob AJ, Hajr EA, Alammar A. Tongue cyst in a neonate:
• Vallecular cysts are a very atypical cause of inspiratory unusual presentation. Cureus. 2020;12(7):e9252
stridor in a newborn. (3) 5. Wang GX, Zhang FZ, Zhao J, et al. Minimally invasive procedure for
• Findings of physical examination can include inspiratory diagnosis and treatment of vallecular cysts in children: review of 156
cases. Eur Arch Otorhinolaryngol. 2020;277(12):3407–3414
stridor, respiratory distress, poor feeding, failure to thrive,
6. Aubin A, Lescanne E, Pondaven S, Merieau-Bakhos E, Bakhos D.
snoring, choking, chronic cough, hoarse cry, cyanosis, Stridor and lingual thyroglossal duct cyst in a newborn. Eur Ann
and croup. (4) Otorhinolaryngol Head Neck Dis. 2011;128(6):321–323
Vol. 25, No. 1 JANUARY 2024 e55
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
INDEX OF SUSPICION IN THE NURSERY
Tachycardia in a Premature Neonate
Megan Carney, MD,* Tamara Kalhan, MD,† Ellis Rochelson, MD*
*Department of Pediatrics, Children’s Hospital at Montefiore, Bronx, NY
†
Department of Pediatrics, Division of Neonatology, Children’s Hospital at Montefiore, Albert Einstein College of Medicine, Bronx, NY
A male infant is delivered at 31 weeks’ gestational age to a 32-year-old woman
with a history of type 1 diabetes mellitus, superimposed preeclampsia, and
smoking history, via urgent C-section indicated for preterm premature rupture
of membranes and concern for placental abruption. In the delivery room, the in-
fant is given continuous positive airway pressure for respiratory distress and
transported to the NICU for prematurity. Laboratory findings on admission are
within normal limits.
In the NICU, the infant is started on total parenteral nutrition, a lipid
injectable emulsion, and human donor milk. Feeds are advanced according
to institutional protocol and fortified with human milk fortifier. On day 6
after birth, the infant is remarkably irritable, and unrelieved by feeding or
pacifying. Physical examination reveals abdominal distention and bloody
stool. Vital signs are notable for sustained tachycardia with a heart rate of
190 to 205 beats/min. Abdominal radiography demonstrates dilation of the
stomach and small bowel loops with pneumatosis intestinalis suggestive of
necrotizing entercolitis. Cardiology is consulted for new-onset persistent
tachycardia.
DISCUSSION
Differential Diagnosis
Tachyarrhythmias can be characterized by narrow or wide QRS complexes.
Wide QRS complexes are generated by slow or uncoordinated ventricular depo-
larization, and can be caused by ventricular tachycardia (VT), supraventricular
tachycardia (SVT) with aberrancy, SVT in an antidromic tachycardia circuit, or
long QT syndrome. (1)(2)(3)(4)(5) This is in contrast to the narrow QRS usually
seen in sinus rhythm that is generated by antegrade conduction through a
healthy His-Purkinje system. Wide complex tachycardia should generally be con-
sidered VT until proven otherwise, though providers must also consider the di-
agnosis of SVT with aberrancy.
AUTHOR DISCLOSURES Drs Carney,
Kalhan, and Rochelson have disclosed no Actual Diagnosis
financial relationships relevant to this The electrocardiogram was notable for wide complex tachycardia with retro-
article. This commentary does not grade conducted P-waves, which are P waves that come after the QRS complex
contain a discussion of an unapproved/
investigative use of a commercial (Fig 1). A second electrocardiogram obtained after the administration of adeno-
product/device. sine (0.1 mg/kg) was notable for loss of retrograde P-wave conduction (Fig 2).
e56 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
Figure 1. Original electrocardiogram with arrows pointing to retrograde P waves.
The persistence of the tachyarrhythmia in the absence of The Condition
retrograde P-waves excluded a reentrant SVT, and the di- VT is a wide-complex arrhythmia originating below the
agnosis of VT was made. Echocardiography demonstrated bundle of His. Identifiable causes of VT in neonates include
normal ventricular anatomy and systolic function. myocarditis, cardiac tumor, hereditary cardiomyopathy, and
Figure 2. Rhythm strip during adenosine administration with black arrows showing retrograde P waves, red arrows showing loss of retrograde P wave
conduction, and blue arrows showing AV dissociation.
Vol. 25, No. 1 JANUARY 2024 e57
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
electrolyte abnormalities. The most common cause, idio- and tends to self-resolve within the first few months of age.
pathic VT, is defined as VT without an identified underlying (12)(13)(14)
cause and often occurs in a child with a structurally normal
heart. (1)(4) However, VT is rarely described in this setting. PATIENT COURSE
Several features on ECG are suggestive of VT, as op- The patient was treated with a lidocaine bolus (1 mg/kg)
posed to SVT with aberrancy. In VT, there may be ventri- and converted to sinus rhythm (Fig 2). He was started on
culoatrial dissociation with a QRS:P ratio greater than 1. an esmolol drip, which was transitioned to propranolol
However, due to healthy retrograde AV node conduction when he was able to tolerate feeds. For treatment of NEC,
in most infants, there may be a one-to-one QRS:P ratio, he continued to receive nothing by mouth and received
which mimics SVT. (3)(6)(7) Other features suggestive of piperacillin-tazobactam for 10 days. Serial abdominal radi-
VT include the presence of fusion beats, in which there is ography was performed until there was a normal bowel
an intermediate QRS morphology resembling a mix of si- gas pattern with no pneumatosis. He was then restarted
nus rhythm and the tachyarrhythmia, and sinus capture on enteral feeds of donor human milk which he tolerated
beats, where a narrow-complex sinus beat is seen during well. The infant did not have any additional episodes of
tachycardia due to capture of antegrade conduction down VT while he was admitted. Propranolol was later discon-
the AV node. (8) Ventriculoatrial dissociation and/or fu- tinued at a follow-up cardiology appointment when the pa-
tient was 5 months old.
sion beats during tachycardia help differentiate VT from
the other types of wide QRS tachycardias. (7)
Lessons for the Clinician
Cases of preterm infants with recurrent SVT having
• When evaluating a neonate with a suspected arrhythmia,
necrotizing enterocolitis (NEC) have been reported, in
clinicians must first assess hemodynamic stability.
which the condition may be due to localized hypoxia in
• Wide QRS complex tachycardia is assumed to be VT in
the intestine. (9)(10) VT decreases cardiac output due to
neonates until proven otherwise.
atrioventricular desynchrony, impaired filling time, and
• If the neonate is hemodynamically stable and if the diag-
decreased stroke volume. (2) In the patient described in
nosis remains unclear, clinicians can give adenosine first
this case, it is possible that VT led to decreased intestinal
to block the atrioventricular node. This can help differenti-
perfusion and subsequent NEC. However, since both pa-
ate between VT and SVT with aberrancy before switching
thologies were discovered concurrently, the causality can- to another class of medications to treat tachyarrhythmia.
not be determined; in fact, the inflammatory state of NEC
may have been the instigating factor that led to the tachy-
arrhythmia. (11) American Board of Pediatrics
Neonatal-Perinatal Content
TREATMENT/MANAGEMENT Specifications
In an infant with VT who is hemodynamically unstable, • Know appropriate management of common
which may be suggested by severe hypotension, low mus- arrhythmias in the fetus and newborn infant,
cle tone, and decreased alertness, defibrillation is indi- and understand the potential complications or
cated. In a hemodynamically stable infant with wide adverse effects of approaches and drugs used.
complex tachycardia, adenosine may be considered to help • Know the physiologic consequences of an ar-
differentiate between VT and SVT with aberrancy. If the rhythmia in a fetus or newborn infant.
adenosine is given during VT, there will be a loss of retro- • Differentiate normal from common abnormal
grade P-waves with no interruption in the tachycardia, as electrocardiographic patterns and rhythms in
occurred in this patient. If the rhythm is SVT, and if aden- the fetus and newborn infant.
osine was given appropriately via rapid intravenous (IV) • Recognize the clinical manifestations, diagno-
push, the tachycardia will usually terminate. (6) Once the sis, and management of infants with perfora-
diagnosis of neonatal VT has been established, if the patient tions of the gastrointestinal tract (including
remains hemodynamically stable, medical management can gastric and intestinal).
be considered. First-line options include IV lidocaine, IV es- • Know the clinical and diagnostic features, eval-
molol, or oral propranolol. (2)(4)(12) Once the infant is in uation, management, and complications of
sinus rhythm, a b-blocker is started. Ultimately, neonatal NEC.
idiopathic ventricular tachycardia has an excellent prognosis
e58 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
References 8. Edhouse J, Morris F. Broad complex tachycardia–part I. BMJ.
2002;324(7339):719–722
1. Escudero CA, Tan RBM, Beath CM, Dalal AS, LaPage MJ, Hill AC. 9. Mecarini F, Comitini F, Bardanzellu F, Neroni P, Fanos V.
Approach to wide complex tachycardia in pediatric patients. CJC Pediatr Neonatal supraventricular tachycardia and necrotizing
Congen Heart Dis. 2022;1(2):60–73 doi: 10.1016/j.cjcpc.2022.02.003 enterocolitis: case report and literature review. Ital J Pediatr.
2. Villain E, Butera G, Bonnet D, Acar P, Aggoun Y, Kachaner J. 2020;46(1):117
Neonatal ventricular tachycardia [article in French]. Arch Mal Coeur
10. Saini J, Moore A, Hodgson K. Necrotising enterocolitis after
Vaiss. 1998;91(5):623–629
supraventricular tachycardia: an unusual precursor to a common
3. Brady WJ, Mattu A, Tabas J, Ferguson JD. The differential diagnosis problem. BMJ Case Rep. 2017;2017:bcr-2017
of wide QRS complex tachycardia. Am J Emerg Med.
2017;35(10):1525–1529 11. De Plaen IG. Inflammatory signaling in necrotizing enterocolitis.
Clin Perinatol. 2013;40(1):109–124
€
4. Jaeggi E, Ohman A. Fetal and neonatal arrhythmias. Clin Perinatol.
2016;43(1):99–112 12. Crosson JE, Callans DJ, Bradley DJ, et al. PACES/HRS expert
consensus statement on the evaluation and management of
5. Ban, J-E. Neonatal arrhythmias: diagnosis, treatment, and clinical
ventricular arrhythmias in the child with a structurally normal heart.
outcome. Korean J Pediatr. 2017;60(11):344–352 doi: 10.3345/
kjp.2017.60.11.344 Heart Rhythm. 2014;11(9):e55–e78
6. Kothari DS, Skinner JR. Neonatal tachycardias: an update. Arch Dis 13. De Rosa G, Butera G, Chessa M, et al. Outcome of newborns with
Child Fetal Neonatal Ed. 2006;91(2):F136–F144 doi: 10.1136/ asymptomatic monomorphic ventricular arrhythmia. Arch Dis Child
adc.2004.049049 Fetal Neonatal Ed. 2006;91(6):F419–F422
7. Katritsis, DG, Brugada J. Differential diagnosis of wide QRS 14. Levin MD, Stephens P, Tanel RE, Vetter VL, Rhodes LA. Ventricular
tachycardias. Arrhythm Electrophysiol Rev. 2020;9(3):155–160 tachycardia in infants with structurally normal heart: a benign
doi: 10.15420/aer.2020.20 disorder. Cardiol Young. 2010;20(6):641–647
Vol. 25, No. 1 JANUARY 2024 e59
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
MATERNAL-FETAL CASE STUDIES
Fetal Injury from Maternal Penetrating
Abdominal Trauma in Pregnancy
Emily Barron, BSPH,* Alison Jeffries, BS,* Sarah Pelton, BS,* Katherine Vogel, BS,* Bobbi J. Byrne, MD†
*Indiana University School of Medicine, Indianapolis, IN
†
Division of Neonatal-Perinatal Medicine, Department of Pediatrics, Indiana University, Indianapolis, IN
CASE PRESENTATION
A 33-year-old gravida 2, para 1-0-0-1 woman at 36 weeks and 4 days’ gestation
was brought to the emergency department by ambulance with multiple stab
wounds to her back and abdomen after having been assaulted at home with a
knife by her teenage son. Upon arrival at the level I trauma center, her Glasgow
Coma Scale score was 15 (fully conscious).
On the primary evaluation by the trauma surgeons, the patient was hemody-
namically stable with an intact airway. A secondary evaluation revealed 2 stab
wounds on her left posterolateral chest and a third wound on her epigastric area
near the fundus (Fig 1). Her blood pressure was 158/102 mm Hg, oxygen satura-
tion on a nonrebreather mask was 93%, and hematocrit was 27%. Maternal
chest radiograph revealed a large left-sided pneumothorax and possible pneumo-
mediastinum, with the heart and mediastinum shifted to the right, as well as
tracks of gas along the maternal left lateral chest wall and neck (Fig 2). Obstet-
rics and neonatology were both urgently consulted. Although a urine drug
screen was positive for benzodiazepines and opiates, it is unclear which medica-
tions were given during transport to the hospital. A confirmatory blood test was
not ordered.
The fetal heart rate tracing on arrival was reassuring without decelerations
and with moderate variability. Obstetric history revealed appropriate prenatal
care with an unremarkable prenatal visit 8 days before presentation to the hospi-
tal. The patient denied any history of domestic violence, intimate partner vio-
lence, or physical abuse.
AUTHOR DISCLOSURES Dr Byrne has
attended meetings, with her co-chair, CASE PROGRESSION
with the support of the American
Academy of Pediatrics Neonatal
The trauma surgeons recommended an exploratory laparotomy via a vertical in-
Resuscitation Program Steering cision to assess for intra-abdominal injury. There was preparation for a possible
Committee and the PLACES Project cesarean delivery by the obstetrical team. After general endotracheal intubation,
Advisory Committee. Mss Barron, Jeffries,
Pelton, and Vogel have disclosed no a chest tube was placed into the left chest for decompression of her pneumotho-
financial relationships relevant to this rax. After anesthesia induction, an assessment of the fetal heart rate demon-
article. This commentary does not strated bradycardia at 70 beats/min; therefore, the interdisciplinary team
contain a discussion of an unapproved/
investigative use of a commercial decided to urgently perform a cesarean delivery. The neonatology team was pre-
product/device. sent with resuscitation equipment and a portable warmer.
e60 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
Figure 1. Location of stab injuries documented during the primary survey. EMS 5 emergency medical services. Originally published in Drexler KA, Quist-
Nelson J, Weil AB. Intimate partner violence and trauma-informed care in pregnancy. Reprinted with permission from Drexler et al. (8).
NEONATAL OUTCOME strips were applied for initial primary closure. The infant
The male infant was delivered emergently at 36 weeks was administered morphine and acetaminophen for pain.
and 4 days’ gestation via cesarean delivery and immedi- He was then transferred to the children’s hospital for
ately evaluated by the neonatology team. Apgar scores surgical evaluation. The wound was inspected, irrigated
were 7 and 8 at 1 and 5 minutes, respectively, and the with normal saline, and reapproximated with sutures. A
birthweight was 3,040 g (76th percentile). The initial barium enema study demonstrated no evidence of rectal
physical examination findings were significant for a 1.5 cm perforation (Fig 3), and brain magnetic resonance imag-
laceration over his left buttock, 2 cm from the anus that ing was normal without evidence of head trauma. He began
was penetrating 1 to 2 cm into the subcutaneous tissue. enteral feedings without difficulty and was transferred back
Direct pressure was applied for hemostasis, and adhesive to the birth hospital, reuniting with his mother, on day 2. He
Vol. 25 No. 1 JANUARY 2024 e61
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
Figure 2. Chest radiograph showing the left pneumothorax and possible left pneumomediastinum.
has remained well at subsequent routine pediatric surveil- The maternal patient was transferred to the ICU where
lance visits through 19 months of age. she received comprehensive care from multiple specialty
teams for 5 days. She received medical care for a residual
MATERNAL OUTCOME pneumothorax post chest tube removal, postoperative il-
During the cesarean delivery, the placenta was delivered in- eus, and a urinary tract infection.
tact without evidence of the knife’s penetration. A laceration The patient was noted to have multiple psychosocial stres-
in the uterine fundus from the stab wound was noted and sors in addition to her operative and postoperative course, in-
repaired. Exploratory laparotomy during trauma surgery re- cluding separation from her newborn son and the police
vealed that the patient had an intact bladder, ovaries, and investigation of the assault by her teenage son. She expressed
fallopian tubes, with no additional visceral injuries. The ma- sadness that she had yet to meet and name her newborn dur-
ternal patient required 2 units of packed red blood cells, ing this critical period of bonding. The initial separation from
fresh frozen plasma, and 4 L of fluid intraoperatively, with her infant son and her own recovery contributed to delays in
an estimated blood loss of 1.5 L of hemoperitoneum. producing an adequate breast milk supply.
e62 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
Figure 3. Barium enema without evidence of rectal perforation. In the image on the left, the small stab wound into the left perineum, adjacent to the in-
fant’s anus, is indicated by the instrument.
The patient experienced flashbacks, anxiety, and nightmares demonstrated in this case, is less common than blunt trauma,
related to the assault. The psychiatry team diagnosed acute but it is associated with higher rates of fetal injury and mortal-
stress disorder; however, the patient declined psychotropic ity. In cases of penetrating abdominal trauma, maternal mor-
medications out of concern for transfer to breast milk. She in- tality is less likely compared with blunt trauma because of
stead opted for outpatient mental health services. Although abdominal visceral displacement and protection by the gravid
her teenage son had initially been excited about the preg- uterus; however, fetal mortality ranges from 40% to 70% be-
nancy, psychiatric symptoms including hallucinations, gran- cause of the high risk associated with preterm delivery or
diose thinking, and paranoia for 2 months leading up to the from direct fetal injury by the penetrating object. (1)(2)
assault tragically affected the family dynamics. The woman The physiologic changes that pregnant patients experi-
reported seeking care at 2 local inpatient psychiatric institu- ence during pregnancy are important for clinicians to un-
tions, both of which discharged her teenage son despite her derstand when caring for a gravid trauma patient. Pregnant
concern that he required further evaluation and interven- patients experience a 45% increase in plasma volume and
tion. During her hospital stay, she worked with law enforce- only an 18% to 30% increase in red cell volume, which re-
ment to ensure charges were not pressed against her son sults in dilutional anemia. Pregnant individuals may lose
and that he received appropriate psychiatric care. Psychiatry !35% of their blood volume before clinical signs of shock
and social work teams provided resources and ensured that are detected. (3) Furthermore, 25% of maternal cardiac out-
she was discharged to a safe environment. At her follow-up put is directed toward the placenta; therefore, penetrating
obstetric and general surgery appointments, she was noted abdominal trauma, particularly if the uterus is penetrated,
to be physically recovering without issue. can quickly lead to hypovolemic shock. (3) Because fetal he-
moglobin binds oxygen with greater affinity than maternal
DISCUSSION hemoglobin, supplemental oxygen should be given to all
The most common nonobstetric cause of mortality during pregnant trauma patients to minimize fetal hypoxia. (2)(4)
pregnancy is trauma. Penetrating abdominal trauma, as If a penetrating injury is present, awareness of expected
Vol. 25 No. 1 JANUARY 2024 e63
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
fundal height based on gestational age is important to as- intraoperative cesarean delivery at the time of exploratory lap-
sess if the injury potentially lacerated the uterus. Lastly, arotomy depending on the clinical situation, gestational age,
inferior vena cava compression from the gravid uterus and injury to the uterus. (4) After delivery, a thorough evalu-
can reduce blood return to the heart by 30%; therefore, ation of the infant is imperative to identify and treat any non-
positioning a pregnant patient in the left lateral decubitus obvious traumatic injuries. Ultimately, beyond the primary
position can help improve circulation. (2) survey, the complexity of trauma in pregnancy requires effi-
The assessment of any trauma patient is standardized to cient medical management and expertise of an interdisciplin-
efficiently identify life-threatening injuries and initiate critical ary team including obstetricians, anesthesiologists, surgeons,
treatments, and this framework is specialized when the and pediatricians.
trauma patient is pregnant. Although this patient’s preg- Healthcare providers should aim to provide universal
nancy was visibly identifiable because of her gestational age, screening for interpersonal violence during pregnancy and
the Society of Obstetricians and Gynecologists of Canada rec- facilitate access to resources and interventions if indicated.
ommends treating every injured female of childbearing age Pregnant women are particularly vulnerable to physical,
as pregnant until proven otherwise because trauma victims emotional, and psychiatric abuse. In pregnancy, more
may be unable to answer questions or may be unaware of their women die from complications of domestic violence than
pregnancy status. (2) In addition to the ABCDE (airway, breath- the combination of the 3 leading obstetric complications
ing and ventilation, circulation, disability, exposure and environ- related to maternal mortality. (6)(7) Prevention of violence
ment) pattern conducted in the primary survey, trauma teams against pregnant women should be advocated for and as-
should determine the gestational age and viability of the fetus sessed like any other pregnancy comorbidity. (8) The US
in pregnant patients. (1) In clinical scenarios involving trauma Preventive Services Task Force recommends the use of
to a pregnant patient, treatment including surgical evaluation, validated screening tools for detecting intimate partner vi-
imaging, and initial medical stabilization should never be de- olence (Table). A large study of pregnant trauma patients
layed or withheld because of the gravid status. (5) found that victims of interpersonal trauma were more
During the secondary survey, fetal heart monitoring likely to have multiple injuries rather than more severe in-
should be initiated, and evidence of placental abruption, juries compared with victims of accidental trauma. (3) The
ruptured membranes, labor, and potential fetal injury study also found that head injuries; contusions of the face,
should be assessed. (1) Urgent involvement of the obstetri- neck, or scalp; and abrasions/friction burns are risk fac-
cal and neonatal teams for multidisciplinary care is criti- tors for interpersonal violence. (3) Beyond detection, ob-
cal. Delivery, even before viability, may be indicated in stetric and pediatric clinicians should offer and advocate
select circumstances to optimize cardiovascular circulation for violence prevention education to minimize exposure to
in a trauma patient in cardiogenic shock. physical conflict where possible. If previous interpersonal
Multiple factors influence the management approach, in- violence is identified, clinicians should make efforts to ob-
cluding the hemodynamic stability of the pregnant patient, jectively document patient responses and examinations in
the extent of the trauma, the gestational age, and the impli- a portion of the electronic medical record system reserved
cations of fetal viability. When treating a pregnant trauma for sensitive information that will not prepopulate in pa-
patient, it is important to maintain maternal hemodynamic tient-accessible portals. By doing this, pregnant individuals
stability as it directly correlates to fetal stability. An obstetrical may be aided in seeking legal protection by providing this
and trauma team must be prepared for the possible need for documentation as corroboration of physical harm. (8)
Table. Screening Tools Recommended for Detecting Intimate Partner Violence
Tool Sensitivity Specificity Description
Humiliation, Afraid, Rape, Kick A 4-item self-report survey based on the Abuse
Instrument (HARK) 81% 95% Assessment Screen
Hurt/Insult/Threaten/Scream A 4-item survey, either self-report or staff-administered,
(HITS) 86%–96% 91%–99% excluding sexual violence
Partner Violence Screen (PVS) A 3-item staff-administered survey assesses violence and
49%–71% 90%–94% current safety, excluding sexual abuse
Slapped, Things, and Threaten A 3-item staff-administered survey assesses violence and
(STaT) 96%–64% 100%–75% threats
Woman Abuse Screening Tool An 8-item self-report survey including emotional and
(WAST) 89%–96% 55% sexual abuse
Adapted with permission from Drexler et al (8).
e64 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
This case illustrates the importance of multidisciplinary
guidelines, benchmarking data, quality
care for a near full-term pregnant patient with a penetrating
improvement).
uterine injury requiring immediate cesarean delivery. Trauma
during pregnancy is not only a leading cause of nonobstetric
maternal mortality, but when directed toward the abdomen, it
can also jeopardize fetal health. Therefore, an understanding
of the use of multidisciplinary evaluation of a trauma patient References
in pregnancy is imperative to properly treat life-threatening in- 1. LA Rosa M, Loaiza S, Zambrano MA, Escobar MF. Trauma in
juries and reduce maternal and fetal mortality. Detection and pregnancy. Clin Obstet Gynecol. 2020;63(2):447–454
education of pregnant women who are at risk of interpersonal 2. Jain V, Chari R, Maslovitz S, et al; Maternal Fetal Medicine
violence should be a priority of health care providers so that Committee. Guidelines for the management of a pregnant trauma
patient. J Obstet Gynaecol Can. 2015;37(6):553–574
trauma in pregnancy can be mitigated.
3. Albini PT, Zakhary B, Edwards SB, Coimbra R, Brenner ML.
Intimate partner violence and pregnancy: nationwide analysis of
injury patterns and risk factors. J Am Coll Surg. 2023;236(1):
American Board of Pediatrics 198–207
Neonatal-Perinatal Content 4. Leshikar DE, Salcedo E. S. Cocanour C. Trauma in Pregnancy. In:
Specifications Moore EE, Feliciano DV, Mattox KL, eds. Trauma. 8th ed. New York,
NY: McGraw-Hill Education; 2017
• Know the significance, interpretation, and
5. American College of Obstetricians and Gynecologists. ACOG Practice
management of abnormalities or changes in fetal Bulletin No 211. Critical care in pregnancy. Obstet Gynecol. 2019;
heart rate patterns during labor including 133:e303–e319
reassuring and nonreassuring and indeterminate 6. El Kady D, Gilbert WM, Xing G, Smith LH. Maternal and neonatal
patterns. outcomes of assaults during pregnancy. Obstet Gynecol. 2005;
• Know the effects on the fetus and/or newborn 105(2):357–363
infant of analgesics and anesthetics administered 7. Lawn RB, Koenen KC. Homicide is a leading cause of death for
pregnant women in US. BMJ. 2022;379:o2499
to the mother during labor.
8. Drexler KA, Quist-Nelson J, Weil AB. Intimate partner violence and
• Know the issues in the organization of perinatal
trauma-informed care in pregnancy. Am J Obstet Gynecol MFM.
care (e.g., regionalization, transport, practice 2022;4(2):100542
ANSWER KEY FOR JANUARY 2024 NEOREVIEWS
Nutritional Needs of the Infant with Bronchopulmonary Dysplasia:
1. A; 2. D; 3.B; 4. C; 5. C
Optimizing Nutrition in Neonates with Kidney Dysfunction:
1. D; 2. D; 3. B; 4. A; 5. C
Vol. 25 No. 1 JANUARY 2024 e65
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
VISUAL DIAGNOSIS
Umbilical Cord Abnormality in a
Monochorionic-Monoamniotic Twin
Pregnancy
Byron S. Maltez, MD,* Maryam Tarsa, MD,† Sandra L. Leibel, MD, MS‡
*School of Medicine, University of California, San Diego, San Diego, CA
†
Department of Obstetrics, Gynecology and Reproductive Sciences, University of California, San Diego, San Diego, CA
‡
Department of Pediatrics, University of California, San Diego, San Diego, CA
THE CASE
This was a monochorionic-monoamniotic (MoMo) twin gestation with abnormal
color Doppler prenatal ultrasound findings at 26 weeks’ gestation.
Prenatal and Birth Histories
• 33-year-old gravida 1, para 0 woman with benign prenatal screening results.
• Spontaneous twin gestation suspected to be MoMo based on lack of visualiza-
tion of the dividing membrane and the spatial relationship between twins A
and B at 12 1/7 weeks’ gestation (Fig 1).
• Fetal survey at 18 weeks’ gestation (anatomy scan): No abnormalities, the placental
cord insertions for twins A and B were in close relationship to each other (Fig. 2).
• Patient was seen beyond 18 weeks’ gestation with outpatient ultrasonography
every 2 weeks to evaluate fetal growth and monitor for twin-to-twin transfu-
sion syndrome.
Presentation (In Utero at 26 weeks’ Gestation)
At 26 weeks’ gestation, a cord abnormality in both twins was detected on routine
Doppler ultrasonography (Fig 3). As a result of this finding, outpatient management in-
creased with twice-weekly antepartum testing and fetal ultrasonography every 2 weeks.
The patient received a course of betamethasone at 28 weeks’ gestation. Fetal growth
and the amniotic fluid volume were normal until 30 weeks’ gestation when twin A
was noted to have growth restriction with an estimated fetal weight at the third percen-
tile. Twin B also demonstrated decreased growth velocity. The woman was admitted to
the hospital for closer monitoring. Over the next week, twin A developed resistive um-
bilical artery Doppler indices with intermittent fetal decelerations. The decision was
made to proceed with delivery via cesarean section at 32 1 2 weeks’ gestation.
AUTHOR DISCLOSURES Drs Maltez,
Tarsa, and Leibel have disclosed no
financial relationships relevant to this DIFFERENTIAL DIAGNOSIS
article. This commentary does not
• Spontaneous antepartum septostomy
contain a discussion of an unapproved/
investigative use of a commercial • Umbilical cord entanglement
product/device. • Umbilical cord knot
e66 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
Figure 1. First trimester transvaginal image at 1 2 1 /7 weeks’ gestation. Figure 3. Second trimester ultrasound scan with color Doppler at 2 6
This image shows that there is no intertwin membrane. AA indicates twin weeks’ gestation showing an umbilical cord anomaly.
A. BB indicates twin B.
ACTUAL DIAGNOSIS Physical Examination (Newborn Day)
Twin A.
Umbilical cord entanglement.
• Vital signs stable, temperature 98.8! F (37.1! C)
• Birthweight 1,400 g (18th percentile), head circumference
PROGRESSION 29 cm (47th percentile), length 42.5 cm (62th percentile).
Both twins emerged vigorous with a spontaneous cry. • Head: Normocephalic and atraumatic; anterior fonta-
Twin A had an Apgar score of 7 and 8 and twin B had an nelle open, soft, and flat
Apgar score of 8 and 9 at 1 and 5 minutes of age, respec- • Oral cavity: Intact palate
tively. Entanglement of the umbilical cords was visible af- • Lungs: Clear, equal breath sounds; no respiratory distress
ter birth (Figs 4 and 5). Both infants were transported to • Cardiovascular: Normal S1 and S2; regular rate and
the NICU receiving continuous positive airway pressure rhythm; no murmurs
(CPAP) at 6 cm H2O with a fraction of inspired oxygen • Abdomen: Soft, nondistended, no masses or organome-
(FIO2) of 0.21. galy; 3-vessel cord
• Genitourinary: Normal preterm female genitalia; patent
anus
Figure 2. First trimester ultrasound scan at 1 8 weeks’ gestation showing Figure 4. Picture of monochorionic-monoamniotic twins soon after delivery
close proximity of the placental cord insertion for twin A and twin B. by caesarean section at 3 2 2 /7 weeks’ gestation with umbilical cords intact.
Vol. 25 No. 1 JANUARY 2024 e67
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
Twin B.
• White blood cell count: 14,200/mL (14.2 × 109/L) with
66% neutrophils and 5% bands
• Hemoglobin and hematocrit: 19.3 g/dL (193 g/L) and
55% (0.55), respectively
• Platelet count: 158 × 103/mL (158 × 109/L)
• Blood glucose: 29 mg/dL (1.6 mmol/L)
• Venous blood gas: pH 7.22, PCO2 60 mm Hg (8 kPa), bi-
carbonate 24.6 mg/dL (24.6 mmol/L), base deficit 3.1
mEq/L (3/1 mmol/L) on CPAP 6, FIO2 21%
Progression
In the NICU, both infants were given intravenous fluid
through an umbilical venous catheter. Both infants also
were treated with caffeine. Twin A was weaned from
Figure 5. Close-up image of the umbilical cord entanglement after
delivery. CPAP to 2 L high-flow nasal cannula (HFNC) at 4 days of
age while twin B was weaned from CPAP to 2 L HFNC at
• Skin: No icterus, birthmarks, petechiae, or other rashes 6 days of age. They both subsequently weaned to room air
• Neurologic: Normal tone and posture for gestational age 8 days after birth. Twin A underwent head ultrasonogra-
phy at 3 days of age, which demonstrated a 2.6-mm left
Twin B. choroid plexus cyst that was noted to have resolved on the
• Vital signs stable, temperature 98.1! F (36.7! C) head ultrasound scan obtained at 30 days of age. Twin B
• Birthweight 1,545 g (30th percentile), head circumference did not have any abnormalities noted on ultrasonography
28 cm (23th percentile), length 42 cm (55th percentile). on either day 3 or 30 days of age. Ophthalmologic screen-
• Head: Normocephalic and atraumatic; anterior fonta- ing for retinopathy and hearing screens showed normal
nelle open, soft, and flat findings.
• Oral cavity: Intact palate The placental pathology showed a hypermature MoMo
• Lungs: Clear, equal breath sounds; no respiratory distress twin placenta (618 g, with A:B ratio of 50%:50%; with in-
• Cardiovascular: Normal S1 and S2; regular rate and creased syncytial knots on both sides of the placenta). Foci
rhythm; no murmurs of villous agglutination were observed on twin A’s placen-
• Abdomen: Soft, nondistended, no masses or organome- tal side and normoblastemia was observed on twin B’s
galy; 3-vessel cord portion of the placenta.
• Genitourinary: Normal preterm female genitalia; patent
anus What the Experts Say
• Skin: No icterus, birthmarks, petechiae, or other rashes Fetal ultrasonography at 18 weeks’ gestation (Fig 2) showed
• Neurologic: Normal tone and posture for gestational 2 umbilical cords with normal color flow; there was no in-
age tertwin membrane and the proximity of the cords suggested
a monoamniotic twin gestation. However, the ultrasound
Laboratory Studies on Admission scan obtained at 26 weeks’ gestation (Fig. 3) was most con-
Twin A. sistent with cord entanglement. This was because of the
• White blood cell count: 8,100/mL (8.1 × 109/L) with 61% chainlike complicated wrapped appearance of the 2 cords.
neutrophils and 0% bands This finding prompted closer monitoring of the pregnancy.
• Hemoglobin and hematocrit: 15.4 g/dL (154 g/L) and Umbilical cord entanglement is believed to be a fairly
46% (0.46), respectively common, if not ubiquitous feature of monoamniotic twin
• Platelet count: 187 × 103/mL (187 × 109/L) pregnancies. (1) Loose cord entanglement without signifi-
• Blood glucose: 23 mg/dL (1.3 mmol/L) cant impingement on blood flow perhaps is not as detri-
• Venous blood gas: pH 7.31, PCO2 49 mm Hg (6.5 kPa), mental to perinatal outcome as tight cord entanglement,
bicarbonate 24.7 mg/dL (24.7 mmol/L), base deficit 1.6 which eventually may lead to fetal death. Given such
mEq/L (1.6 mmol/L) on CPAP 6, FIO2 0.21 unique yet common characteristics of monoamniotic twin
e68 NeoReviews
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
pregnancies and the risk of fetal loss, inpatient and close
guidelines for resuscitation and outcomes are
fetal monitoring is a common practice in this scenario. Fe-
dependent on the gestational age and birth-
tal loss due to cord entanglement has been thought to be
weight at delivery.
a gradual rather than a sudden event. (2) Hence, the fre-
quency of fetal monitoring should be tailored based on
gestational age and interpretation of non-stress testing,
which will help determine the date of delivery.
American Board of Pediatrics
Rossi et al found that cord entanglement could be con-
sidered a minor complication of MoMo twin pregnancies,
Neonatal-Perinatal Content
citing an 84% survival rate of both twins and a 90% sur- Specifications
vival rate of at least 1 twin. (3) The mere presence of cord • Know the general principles, applications, and
entanglement does not in and of itself contribute to neo- limitations of ultrasonography, including Doppler
natal morbidity and mortality. Umbilical cord entanglement blood flow measurements, in assessment of fetal
was historically believed to be the cause of intrauterine fetal conditions and well-being.
death in monoamniotic pregnancies, however, given the • Know the rationale, interpretation, and limitations
ubiquitous nature of this finding, other mechanisms are of maternal detection of fetal movement, of the
likely at play. (4) Despite the high incidence of cord entan- biophysical profile, the non-stress test, and the
glement in MoMo twins, Chitrit et al found that neonatal contraction stress test as means of assessing fetal
outcomes were largely good for affected infants. (5) A retro- well-being.
spective study in England demonstrated that 74% of MoMo • Know the potential fetal complications of multiple
twins required NICU admission with respiratory distress gestation such as cord problems, twin-twin
syndrome as the most common diagnosis as a result of pre- transfusion, “stuck twin,” conjoined twins, etc.
term birth. Eighty-six percent of MoMo twins had a defini- • Know how to evaluate fetal growth rate and fetal
tive diagnosis of cord entanglement postpartum. (6) growth restriction and the management of fetal
The lack of a dividing membrane between twins A and growth restriction.
B had been confirmed on multiple ultrasound scans in the
early first trimester, which makes spontaneous antepar-
tum septostomy very unlikely. Umbilical cord knots can
References
be seen in singleton fetuses while cord entanglement re-
1. Dias T, Mahsud-Dornan S, Bhide A, Papageorghiou AT, Thilaganathan
quires the presence of 2 or more cords in utero. Hence,
B. Cord entanglement and perinatal outcome in monoamniotic twin
the ultrasound cord finding was mostly consistent with pregnancies. Ultrasound Obstet Gynecol. 2010;35(2):201–204
the existence of cord entanglement. 2. Post A, Heyborne K. Managing monoamniotic twin pregnancies. Clin
Obstet Gynecol. 2015;58(3):643–653
3. Rossi AC, Prefumo F. Impact of cord entanglement on perinatal
Summary outcome of monoamniotic twins: a systematic review of the literature.
Ultrasound Obstet Gynecol. 2013;41(2):131–135
• Cord entanglement is a common occurrence in
4. Lewi L. Cord entanglement in monoamniotic twins: does it really
MoMo twin gestations. matter? Ultrasound Obstet Gynecol. 2010;35(2):139–141
• Routine prenatal monitoring is required for de- 5. Chitrit Y, Korb D, Morin C, Schmitz T, Oury J-F, Sibony O. Perinatal
tecting changes in fetal growth and to decrease mortality and morbidity, timing and route of delivery in
the risk of fetal death; monitoring should be tai- monoamniotic twin pregnancies: a retrospective cohort study. Arch
Gynecol Obstet. 2021;303(3):685–693
lored based on gestational age.
6. Glinianaia SV, Rankin J, Khalil A, et al. Prevalence, antenatal
• Postnatal management of MoMo twin newborns management and perinatal outcome of monochorionic monoamniotic
with cord entanglement should follow routine twin pregnancy: a collaborative multicenter study in England, 2000-
2013. Ultrasound Obstet Gynecol. 2019;53(2):184–192
Vol. 25 No. 1 JANUARY 2024 e69
Downloaded from /neoreviews/issue/25/1
by Almoosa Hospital user
You might also like
- Neoreviews: Moc MocDocument76 pagesNeoreviews: Moc Mocsalamred100% (2)
- NeoReviews February 2024Document58 pagesNeoReviews February 2024utinhies1No ratings yet
- Phonics Level ADocument421 pagesPhonics Level AEman Maher100% (2)
- Pir December 2023Document62 pagesPir December 2023ceciliagandulfoNo ratings yet
- AEF 5 Students BookDocument177 pagesAEF 5 Students Bookcamila freitas100% (1)
- Activity Book For 3-4 Years Children PDFDocument54 pagesActivity Book For 3-4 Years Children PDFMarietta Mosolygó100% (1)
- Activity Book For 3-4 Years ChildrenDocument54 pagesActivity Book For 3-4 Years ChildrenMuhammad Zakria YahyaNo ratings yet
- 70 Play Activities Thinking Selfregulation Learning BehaviorDocument199 pages70 Play Activities Thinking Selfregulation Learning BehaviorRyan FinleyNo ratings yet
- M10 RecurrentSelDocument28 pagesM10 RecurrentSelFilipe FariasNo ratings yet
- Consumer Decision Making:: Back To The Future? Chocolate Lounges Taste Sweet SuccessDocument7 pagesConsumer Decision Making:: Back To The Future? Chocolate Lounges Taste Sweet SuccessmanpreetklerNo ratings yet
- Juvenile DelinquencyDocument9 pagesJuvenile DelinquencyChayanNo ratings yet
- Activity Book For 3-4 Years ChildrenDocument49 pagesActivity Book For 3-4 Years Childrenlokiloki.svsNo ratings yet
- MBOYONG DEWI SRI - AlangalangkumitirDocument5 pagesMBOYONG DEWI SRI - AlangalangkumitirafifbawasantosaNo ratings yet
- Story Topic Brainstorming WorksheetDocument3 pagesStory Topic Brainstorming Worksheetsara manarhaqNo ratings yet
- Dok Baru 2018-08-02 - 1 (2 Files Merged)Document3 pagesDok Baru 2018-08-02 - 1 (2 Files Merged)dina alvionitaNo ratings yet
- Full Download Ebook PDF Health Promotion Programs From Theory To Practice 2nd Edition PDFDocument41 pagesFull Download Ebook PDF Health Promotion Programs From Theory To Practice 2nd Edition PDFelizabeth.shannon124100% (36)
- Add The EndingDocument1 pageAdd The Endingapi-595567391No ratings yet
- Dig It Digital: Presented By: Group 11 Manvi Bolia Mohdmahir Shaikh Nancy Gupta Nehal Jain Nishita Arora Piyush HirwaniDocument13 pagesDig It Digital: Presented By: Group 11 Manvi Bolia Mohdmahir Shaikh Nancy Gupta Nehal Jain Nishita Arora Piyush HirwaniManvi BoliaNo ratings yet
- 5 WsDocument2 pages5 WsJulius GaviolaNo ratings yet
- Elementary Reading Attitude Survey: "Garfield"© Paws, Inc. All Rights ReservedDocument5 pagesElementary Reading Attitude Survey: "Garfield"© Paws, Inc. All Rights Reservedapi-548626684No ratings yet
- Dars e NizamiDocument8 pagesDars e NizamiNaveed Ahmed Butt100% (1)
- Dars e NizamiDocument8 pagesDars e NizamiNaveed Ahmed ButtNo ratings yet
- Ebook PDF Teaching Primary Science Constructively 6th Australia PDFDocument41 pagesEbook PDF Teaching Primary Science Constructively 6th Australia PDFdanny.bueno216100% (41)
- Inferno Unitool V1.5.7 Crack Setup Free Download: What'S Hot!Document1 pageInferno Unitool V1.5.7 Crack Setup Free Download: What'S Hot!PepeNo ratings yet
- NGIRIT IKU PERLU - AlangalangkumitirDocument5 pagesNGIRIT IKU PERLU - AlangalangkumitirafifbawasantosaNo ratings yet
- FINALDocument1 pageFINALDivija PampanaNo ratings yet
- Assessment of Technology Infrastructure in Native CommunitiesDocument126 pagesAssessment of Technology Infrastructure in Native CommunitiesStimulatingBroadband.comNo ratings yet
- Espina and Malaran Mil ReportDocument20 pagesEspina and Malaran Mil ReportEduardo BasonNo ratings yet
- PHAR 2101L PreFinals TransDocument5 pagesPHAR 2101L PreFinals TransJoseph Basilan UrmenetaNo ratings yet
- Parents As Partners: Bonnie Meisel M.Ed., NBCT Susan Ray M.Ed., LPC, NBCT Norman, OklahomaDocument19 pagesParents As Partners: Bonnie Meisel M.Ed., NBCT Susan Ray M.Ed., LPC, NBCT Norman, OklahomasrayokNo ratings yet
- BEDHAYAN DURADASIH - AlangalangkumitirDocument6 pagesBEDHAYAN DURADASIH - AlangalangkumitirafifbawasantosaNo ratings yet
- Hero On A Mission WorkbookDocument13 pagesHero On A Mission WorkbooksomawritescopyNo ratings yet
- ISCA Mnemonic Codes PDFDocument44 pagesISCA Mnemonic Codes PDFHimanshu Jeerawala100% (2)
- Learning ContractDocument3 pagesLearning Contractapi-3832766No ratings yet
- StrategiesDocument7 pagesStrategiesShakambhari KumariNo ratings yet
- Title: General Physical Traits Character Traits InterestsDocument1 pageTitle: General Physical Traits Character Traits InterestsAnonymous omgkwYNo ratings yet
- Ele Unit4 Extension PDFDocument2 pagesEle Unit4 Extension PDFNatalia ZapataNo ratings yet
- 20 Conrad Volunteer RecruitmentDocument14 pages20 Conrad Volunteer RecruitmentmhetfieldNo ratings yet
- Quality ControlDocument19 pagesQuality ControlAisha AbuzgaiaNo ratings yet
- Client Presentation-FinalDocument16 pagesClient Presentation-Finalapi-459051817No ratings yet
- Jean A. Killebrew: 5905 Rosemead Boulevard Unit # 5 Pico Rivera, CA 90660 562 338-2249 CellDocument3 pagesJean A. Killebrew: 5905 Rosemead Boulevard Unit # 5 Pico Rivera, CA 90660 562 338-2249 Cellapi-23314319No ratings yet
- Fieldsr 341 Rhonda Fields ResumeDocument4 pagesFieldsr 341 Rhonda Fields Resumeapi-23753839No ratings yet
- Porfolio Assessment 011Document2 pagesPorfolio Assessment 011ML BelanoNo ratings yet
- Samantha Nunn, Research Facilitator, Cambridgeshire Community Services NHS Trust (CCS NHST)Document1 pageSamantha Nunn, Research Facilitator, Cambridgeshire Community Services NHS Trust (CCS NHST)api-286232866No ratings yet
- You Are Here: A Year of Being and BecomingDocument21 pagesYou Are Here: A Year of Being and BecomingAnthony LNo ratings yet
- DMBT2 Group3 SecDDocument14 pagesDMBT2 Group3 SecDSuyash SarafNo ratings yet
- PDF Created With Fineprint Pdffactory Trial VersionDocument385 pagesPDF Created With Fineprint Pdffactory Trial Versionbaghloul ahmedNo ratings yet
- Introduction To Marketing Management: Chapter 5 & 6Document26 pagesIntroduction To Marketing Management: Chapter 5 & 6samitashresthaNo ratings yet
- Lessons From The Early Christians: WeqveDocument52 pagesLessons From The Early Christians: WeqveBrian S. MarksNo ratings yet
- 6 Month Plan Sample OnlyDocument6 pages6 Month Plan Sample OnlyLeslie Buned CaragNo ratings yet
- Consumer Involvement: Anshaj Goyal (2121748) Aryan Paul (2121743)Document12 pagesConsumer Involvement: Anshaj Goyal (2121748) Aryan Paul (2121743)Aryan PaulNo ratings yet
- Silhouette 6.0 Silhouette v6 UserGuideDocument770 pagesSilhouette 6.0 Silhouette v6 UserGuideSue Uaz Jorje50% (2)
- Gr1-2 Sentence Missing SubjectsDocument2 pagesGr1-2 Sentence Missing Subjectsabbyreader07100% (1)
- 5 Star Client PoliDocument4 pages5 Star Client Poligpurcar100% (1)
- Session 13 Administering+questionnaireDocument16 pagesSession 13 Administering+questionnaireAliyan RazzaqNo ratings yet
- Aero Yoga Flight Manual, Jason Nemer & Jenny Sauer-KleinDocument134 pagesAero Yoga Flight Manual, Jason Nemer & Jenny Sauer-KleinFernando González ClaraNo ratings yet
- Materi Kuliah AKI-, Pert. Ke 1Document17 pagesMateri Kuliah AKI-, Pert. Ke 1Deva Ayu lestariNo ratings yet
- Child GuidanceDocument13 pagesChild GuidanceBetsy CritesNo ratings yet
- DuchenneDocument13 pagesDuchennejenika studiesNo ratings yet
- Physician CertificationDocument1 pagePhysician CertificationcwadotorgNo ratings yet
- Epi Brochure (Calalang)Document2 pagesEpi Brochure (Calalang)Nickaela CalalangNo ratings yet
- Medical Examination Rules 2005Document12 pagesMedical Examination Rules 2005MutuajmNo ratings yet
- Juni Save Root Pulp J LSTR LDocument6 pagesJuni Save Root Pulp J LSTR LAntony SebastianNo ratings yet
- Biographic Data: Nursing Health HistoryDocument14 pagesBiographic Data: Nursing Health HistoryMae Jane AuzaNo ratings yet
- 5 Malado Guillain Barre SyndromeDocument7 pages5 Malado Guillain Barre SyndromeAllan CastroNo ratings yet
- Vitamin B6 Deficiency As A Cause of Polyneuropathy in POEMS Syndrome Rapid Recovery With Supplementation in Two CasesDocument7 pagesVitamin B6 Deficiency As A Cause of Polyneuropathy in POEMS Syndrome Rapid Recovery With Supplementation in Two CasesYaseen MohamnadNo ratings yet
- M-i-M For DME Matrix-In-A-Matrix Technique For Deep Margin ElevationDocument5 pagesM-i-M For DME Matrix-In-A-Matrix Technique For Deep Margin Elevationfernando vicente100% (1)
- Community Health Nursing I (Individual and Family) : Prepared By: Mrs. Lavinia T. Malabuyoc, MAN, R.NDocument48 pagesCommunity Health Nursing I (Individual and Family) : Prepared By: Mrs. Lavinia T. Malabuyoc, MAN, R.NLerma Pagcaliwangan100% (1)
- Organ DonationDocument44 pagesOrgan DonationKlause Kenze Kabil50% (2)
- What Is The Difference Between Tendonitis, Tendinosis, and TendinopathyDocument3 pagesWhat Is The Difference Between Tendonitis, Tendinosis, and TendinopathySylvia GraceNo ratings yet
- Double Anaerobic Coverage Guideline AgainstDocument2 pagesDouble Anaerobic Coverage Guideline AgainstioanaNo ratings yet
- Retic MethodDocument18 pagesRetic MethodFaty DearNo ratings yet
- The Direct Antiglobulin Test: Indications, Interpretation, and PitfallsDocument6 pagesThe Direct Antiglobulin Test: Indications, Interpretation, and Pitfallsabbhyasa5206No ratings yet
- Hepatitis ADocument11 pagesHepatitis AIGA ABRAHAMNo ratings yet
- Ican BrochureDocument16 pagesIcan BrochureAbhishek ShatagopachariNo ratings yet
- Case Study 1Document4 pagesCase Study 1api-271668042No ratings yet
- Healthcare Insurance From A To Z - AnderzorgDocument86 pagesHealthcare Insurance From A To Z - AnderzorgJeremySitorusNo ratings yet
- Journal Club PDADocument30 pagesJournal Club PDASnehal PatilNo ratings yet
- Milk Thistle - Product PresentationDocument14 pagesMilk Thistle - Product PresentationAABHIRAMNo ratings yet
- Special Pathology Viva Questions by AMS 46Document32 pagesSpecial Pathology Viva Questions by AMS 46Mohan Dass100% (1)
- Drowning - Gary WilliamsDocument48 pagesDrowning - Gary WilliamsFitri Amelia RizkiNo ratings yet
- Diagnosis Chinese MedicineDocument215 pagesDiagnosis Chinese Medicinealejandro jeanNo ratings yet
- AMG Curs 3Document2 pagesAMG Curs 3Maria PalNo ratings yet
- Demodex Infestation Requires Immediate, Aggressive Treatment by Doctor, Patient - Primary Care Optometry NewsDocument5 pagesDemodex Infestation Requires Immediate, Aggressive Treatment by Doctor, Patient - Primary Care Optometry NewsΔιονύσης ΦιοραβάντεςNo ratings yet
- WedDocument6 pagesWedPratiwi IkaNo ratings yet
- Effectiveness of Structured Teaching Program On Self-Medication Knowledge and Its Adverse Effects Among Middle Age Adults Residing in Lalitpur, NepalDocument8 pagesEffectiveness of Structured Teaching Program On Self-Medication Knowledge and Its Adverse Effects Among Middle Age Adults Residing in Lalitpur, NepalIJAR JOURNALNo ratings yet
- Slle Examination Content Guideline - March 05 PDFDocument16 pagesSlle Examination Content Guideline - March 05 PDFYahya Alkamali100% (1)
- Septic ShockDocument26 pagesSeptic ShockIma SoniaNo ratings yet