Professional Documents
Culture Documents
Ch14 Quick Check 3
Uploaded by
Канат Тютенов0 ratings0% found this document useful (0 votes)
12 views1 pageCopyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
12 views1 pageCh14 Quick Check 3
Uploaded by
Канат ТютеновCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
Westminster H.S.
AP Chemistry Name __________________________
Date ___/___/___ Period ___
14 Acids and Bases
QUICK CHECK 3
pH’s are Logarithmic
Solution A has a pH of 3. Solution B has a pH of 6. Which solution is more acidic? _____
How many times more acidic is the more acidic solution? ___
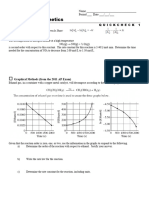
ICE Box with a Twist
A 0.10 M solution of HF has a pH of 2.10. Calculate the Ka of HF.
Strengths of Acids
Consider: HClO2, HBrO2, HIO2. Rank them from weakest to strongest.
Weakest Strongest
Consider: HBrO, HBrO2, HBrO3. Rank them from weakest to strongest.
Weakest Strongest
Consider: HCl, HBr, HI. Rank them from weakest to strongest.
Weakest Strongest
Diprotic Acid Calculations
Sulfurous acid, H2SO3, is a diprotic acid. Ka1 = 1.5 x 10-5; Ka2 = 1.0 x 10-7
What is the [SO32-] in a 0.150 M solution of H2SO3? ___________
Calculate the pH of a 0.150 M solution of H2SO3. _______
You might also like
- Name - Chemistry I-2 HONORS Study Guide For Acids and Bases (Chapter 17)Document2 pagesName - Chemistry I-2 HONORS Study Guide For Acids and Bases (Chapter 17)api-3706290No ratings yet
- Acid Base ReviewDocument4 pagesAcid Base ReviewJeffrey HuangNo ratings yet
- Ch14 Salt HydrolysisDocument1 pageCh14 Salt HydrolysisКанат ТютеновNo ratings yet
- Acid Base Concepts (Quiz With Answers)Document12 pagesAcid Base Concepts (Quiz With Answers)heylinssNo ratings yet
- CH 6 - Acids & BasesDocument71 pagesCH 6 - Acids & BasesCharbel RahmeNo ratings yet
- CH186 Acid-Base Exam Questions From Spring 2001 SemesterDocument6 pagesCH186 Acid-Base Exam Questions From Spring 2001 SemesterArda RahmainiNo ratings yet
- Laporan Praktikum Kimor Uas IndoDocument10 pagesLaporan Praktikum Kimor Uas IndoRisyaUtavianiNo ratings yet
- 004 2024 Nurullah Aulia Sugiarti Rombel 01 Tugas Ke 01Document85 pages004 2024 Nurullah Aulia Sugiarti Rombel 01 Tugas Ke 01eliNo ratings yet
- Acid Base Intro Powerpoint 2020Document35 pagesAcid Base Intro Powerpoint 2020JulesNo ratings yet
- 029 3105 Bunga Rombel1 Tugaske01Document81 pages029 3105 Bunga Rombel1 Tugaske01Corinne SandersNo ratings yet
- Requirements For A Titrimetric ReactionDocument11 pagesRequirements For A Titrimetric ReactionAbdo RaafatNo ratings yet
- Johnston Chapter 17 NotesDocument114 pagesJohnston Chapter 17 NotesRayna RamsinghNo ratings yet
- PhcalculationswkstDocument2 pagesPhcalculationswkstwayne.ilearnacadhkNo ratings yet
- Tut-Acids and BasesDocument30 pagesTut-Acids and BasesThabelo NgwenyaNo ratings yet
- The PH Scale: Activity 2.6Document6 pagesThe PH Scale: Activity 2.6Nigatu MAmoNo ratings yet
- Chem 12Document2 pagesChem 12samuel asefaNo ratings yet
- Acid-Base Chemistry. Extra Practice Problems General Types/Groups of ProblemsDocument13 pagesAcid-Base Chemistry. Extra Practice Problems General Types/Groups of ProblemsYeabisraNo ratings yet
- Acids and BasesDocument28 pagesAcids and BasesAlaric IskandarNo ratings yet
- Ionic EquilibriaDocument2 pagesIonic EquilibriaAnonymous ZVYEN6rBNNo ratings yet
- Acids & Bases Lecture NotesDocument51 pagesAcids & Bases Lecture NotesTahir Hussain100% (1)
- Acid Base Note01-10 StudentDocument28 pagesAcid Base Note01-10 Studentc_66hsia7505No ratings yet
- CH 16 Acid and Bases QuizDocument4 pagesCH 16 Acid and Bases QuizAindrila KaziNo ratings yet
- Test2 Ch17a Acid-Base Practice Problems PDFDocument12 pagesTest2 Ch17a Acid-Base Practice Problems PDFRaphael CastilloNo ratings yet
- 1BAcid Base Worksheet1Document4 pages1BAcid Base Worksheet1Pramudith LiyanageNo ratings yet
- WS4. Lewis Bronsted-Lowry Acids Worksheet (HL)Document4 pagesWS4. Lewis Bronsted-Lowry Acids Worksheet (HL)Yuvraj GuptaNo ratings yet
- GenChem2 Q4 MELC 7-9 Week-5Document7 pagesGenChem2 Q4 MELC 7-9 Week-5BSED FIL 1- Ashley Romarie A. LactaotaoNo ratings yet
- Biochemistry Quiz - Lab - Expt 1Document2 pagesBiochemistry Quiz - Lab - Expt 1JEAN I MAGLAQUENo ratings yet
- 25 Buffers - SDocument6 pages25 Buffers - SLeia JonesNo ratings yet
- Acid-Base Practice ProblemsDocument12 pagesAcid-Base Practice ProblemsDAKSH CHETAN HATHINo ratings yet
- 2016, Chem. Unit 1&2 Practice QuestionDocument12 pages2016, Chem. Unit 1&2 Practice Questionabdilema16No ratings yet
- Natural IndicatorDocument4 pagesNatural Indicatoralezandra caringalNo ratings yet
- Acid and BaseDocument2 pagesAcid and BaseMaraNo ratings yet
- Acid-Base Physiology: Kidney and Body FluidsDocument9 pagesAcid-Base Physiology: Kidney and Body Fluidsgasman2003No ratings yet
- Chapter 18 AssessmentDocument23 pagesChapter 18 Assessmentmilemike67% (6)
- Workbook - AcidsDocument132 pagesWorkbook - AcidsAgustina Itin100% (1)
- Acid-Base Equilibrium - Part 1 - ProblemsDocument3 pagesAcid-Base Equilibrium - Part 1 - ProblemsCraig Juliene NavaltaNo ratings yet
- Gen Chem Practice Problems Ch10, 18 & Buffers f08Document6 pagesGen Chem Practice Problems Ch10, 18 & Buffers f08Anonymous rFIshYyNo ratings yet
- 2017 Unit 2 Chemistry KTT 2 Acids and Bases - Question Book PDFDocument10 pages2017 Unit 2 Chemistry KTT 2 Acids and Bases - Question Book PDFfrank sinatraaNo ratings yet
- Problem Set: Acid-Base EquilibriaDocument8 pagesProblem Set: Acid-Base EquilibriaPamie Penelope BayogaNo ratings yet
- Acids and Bases WorksheetDocument2 pagesAcids and Bases WorksheetChristian Josef AvelinoNo ratings yet
- PH N pOH PDFDocument9 pagesPH N pOH PDFEnsette TroykeNo ratings yet
- 05 - The Chemistry of Acids and Bases Complete - RevisedDocument63 pages05 - The Chemistry of Acids and Bases Complete - RevisedKabesang TalesNo ratings yet
- AP Unit9 Worksheet AnswersDocument5 pagesAP Unit9 Worksheet AnswersAAVANINo ratings yet
- SL Paper 1: Incorrect For A 0.10 Mol DM HCOOH Solution?Document11 pagesSL Paper 1: Incorrect For A 0.10 Mol DM HCOOH Solution?Sai SuhasNo ratings yet
- Experiment 15. Acids, Bases, Salts and Buffers: Objective: 1. To Understand An Acid-Base ReactionDocument6 pagesExperiment 15. Acids, Bases, Salts and Buffers: Objective: 1. To Understand An Acid-Base ReactionNathan Ray AlimNo ratings yet
- TitrationSE PDFDocument7 pagesTitrationSE PDFAmaan Allana33% (3)
- General Chemistry Q4 M3Document14 pagesGeneral Chemistry Q4 M3Brhian DianaNo ratings yet
- Chapter10 (Acids and Bases)Document38 pagesChapter10 (Acids and Bases)Shir0 NobiNo ratings yet
- Exp 4Document5 pagesExp 4Crystal VangelineNo ratings yet
- Chapter 14 Notes-Acids and Bases Bronsted - Lowry Theory: Proton Donors Proton AcceptorsDocument7 pagesChapter 14 Notes-Acids and Bases Bronsted - Lowry Theory: Proton Donors Proton AcceptorsSarah MudaliarNo ratings yet
- Structure of Biological Macromolecules: Chemical EquilibriaDocument27 pagesStructure of Biological Macromolecules: Chemical EquilibriaPutterNo ratings yet
- Acids Are Sour Tasting: Arrhenius AcidDocument28 pagesAcids Are Sour Tasting: Arrhenius AcidDrAmit VermaNo ratings yet
- Lab 2 - PH and Buffers - 2022Document15 pagesLab 2 - PH and Buffers - 2022Kazi AnjumNo ratings yet
- PHCM223 Midterm Revision SS16 443Document20 pagesPHCM223 Midterm Revision SS16 443Michelle MenciasNo ratings yet
- Buffer Solutions.: Ass. Prof. I. R. BekusDocument27 pagesBuffer Solutions.: Ass. Prof. I. R. BekusNanda ThyarezaNo ratings yet
- Prep For FT Hydrolysis and BufferDocument10 pagesPrep For FT Hydrolysis and BufferMutiara DhitaNo ratings yet
- Chemistry in Anhydrous, Prototropic Solvents: Inorganic Chemistry in Liquid Hydrogen Cyanide and Liquid Hydrogen FluorideFrom EverandChemistry in Anhydrous, Prototropic Solvents: Inorganic Chemistry in Liquid Hydrogen Cyanide and Liquid Hydrogen FluorideRating: 1 out of 5 stars1/5 (1)
- Two Points AnswersDocument2 pagesTwo Points AnswersКанат ТютеновNo ratings yet
- Chapter 7 FULL PresentationDocument134 pagesChapter 7 FULL PresentationКанат ТютеновNo ratings yet
- Quick Check 1 and 2Document2 pagesQuick Check 1 and 2Канат ТютеновNo ratings yet
- Station Review AnswersDocument5 pagesStation Review AnswersКанат ТютеновNo ratings yet
- IMF NotesDocument23 pagesIMF NotesКанат ТютеновNo ratings yet
- Quick Check 1Document1 pageQuick Check 1Канат ТютеновNo ratings yet
- IMF WorksheetDocument3 pagesIMF WorksheetКанат ТютеновNo ratings yet
- Introduction To EnergyDocument2 pagesIntroduction To EnergyКанат ТютеновNo ratings yet
- Le Chateliers Principle Practice 2Document2 pagesLe Chateliers Principle Practice 2Канат ТютеновNo ratings yet
- Heating CurveDocument1 pageHeating CurveКанат ТютеновNo ratings yet
- Hess S Law NotesDocument2 pagesHess S Law NotesКанат ТютеновNo ratings yet
- Chapter 7 Quick Check 3Document1 pageChapter 7 Quick Check 3Канат ТютеновNo ratings yet
- Energies of Solution FormationDocument2 pagesEnergies of Solution FormationКанат ТютеновNo ratings yet
- End Point Calculations AnswersDocument2 pagesEnd Point Calculations AnswersКанат ТютеновNo ratings yet
- End Point CalculationsDocument2 pagesEnd Point CalculationsКанат ТютеновNo ratings yet
- IMF TrendsDocument1 pageIMF TrendsКанат ТютеновNo ratings yet
- Chapter 13 Study QuestionsDocument2 pagesChapter 13 Study QuestionsКанат ТютеновNo ratings yet
- Ch14 PH CalculationsDocument1 pageCh14 PH CalculationsКанат ТютеновNo ratings yet
- AP PPT CH 11Document68 pagesAP PPT CH 11Канат ТютеновNo ratings yet
- Buffer CalculationsDocument2 pagesBuffer CalculationsКанат ТютеновNo ratings yet
- AP PPT CH 9 AP OnlyDocument42 pagesAP PPT CH 9 AP OnlyКанат ТютеновNo ratings yet
- AP PPT CH 2 AP OnlyDocument26 pagesAP PPT CH 2 AP OnlyКанат ТютеновNo ratings yet