Professional Documents
Culture Documents
Buffer Calculations
Uploaded by
Канат Тютенов0 ratings0% found this document useful (0 votes)
34 views2 pagesThis document provides instructions for solving two buffer problems involving calculations of hydrogen ion concentration. The first problem involves mixing acetic acid solution and sodium hydroxide solution to form a buffer solution. The second problem adds hydrochloric acid to the buffer solution formed in the first problem to calculate the new hydrogen ion concentration. Both problems involve setting up mole and volume calculations, writing neutralization and equilibrium reactions, and using the equilibrium expression and given Ka value to solve for [H+].
Original Description:
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document provides instructions for solving two buffer problems involving calculations of hydrogen ion concentration. The first problem involves mixing acetic acid solution and sodium hydroxide solution to form a buffer solution. The second problem adds hydrochloric acid to the buffer solution formed in the first problem to calculate the new hydrogen ion concentration. Both problems involve setting up mole and volume calculations, writing neutralization and equilibrium reactions, and using the equilibrium expression and given Ka value to solve for [H+].
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
34 views2 pagesBuffer Calculations
Uploaded by
Канат ТютеновThis document provides instructions for solving two buffer problems involving calculations of hydrogen ion concentration. The first problem involves mixing acetic acid solution and sodium hydroxide solution to form a buffer solution. The second problem adds hydrochloric acid to the buffer solution formed in the first problem to calculate the new hydrogen ion concentration. Both problems involve setting up mole and volume calculations, writing neutralization and equilibrium reactions, and using the equilibrium expression and given Ka value to solve for [H+].
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
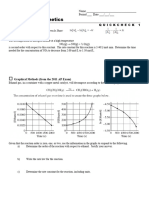
Westminster H.S.
AP Chemistry Name ___________________________________
Period ___ Date ___/___/___
Acid-Base Equilibria
BUFFER CALCULATIONS
The weak acid, acetic acid, has the formula, HC2H3O2. Ka acetic acid = 1.8x10-5
(a) A buffer solution is prepared by adding 0.10 Liter of 2.0 molar acetic acid solution to 0.10 Liter of a 1.0
molar sodium hydroxide solution. Compute the hydrogen ion concentration of the buffer solution.
PROBLEM BREAKDOWN:
(i) Calculate moles of HC2H3O2.
(ii) Calculate moles of OH-.
(iii) What is the total volume (in Liters)?
(iv)Fill in this NEUTRALIZATION chart using Molarities
HC2H3O2 + OH- H2O(l) + C2H3O2-
(v) Fill in this EQUILIBRIUM chart:
HC2H3O2 + H2O(l) H3O+ + C2H3O2-
(vi)Write the equilibrium expression for HC2H3O2.
Substitute the equilibrium values into the expression and compute the hydrogen ion concentration.
Ka acetic acid = 1.8x10-5
(b) Suppose that 0.010 Liter of 0.50 M hydrochloric acid is added to 0.20 Liter of the buffer prepared in (a).
Compute the hydrogen ion concentration of the resulting solution.
PROBLEM BREAKDOWN:
(i) Calculate moles of HC2H3O2 and C2H3O2-.
(ii) Calculate moles of H+.
(iii) What is the total volume (in Liters)?
(iv)Fill in this chart using Molarities
C2H3O2- + H+ HC2H3O2
(v) Fill in this chart:
HC2H3O2 + H2O(l) H3O+ + C2H3O2-
(vi)Write the equilibrium expression for HC2H3O2.
Substitute the equilibrium values into the expression and compute the hydrogen ion concentration.
You might also like
- Tutorial 3 Chapter 3Document2 pagesTutorial 3 Chapter 3Nor ShasalbilaNo ratings yet
- Ionic Equilibria + Group 17 QuestionsDocument25 pagesIonic Equilibria + Group 17 QuestionsWisley YuanShenNo ratings yet
- Applications of Acids Bases Review - KeyDocument11 pagesApplications of Acids Bases Review - Keyapi-90184144No ratings yet
- Part IV 2019 Updated QBDocument8 pagesPart IV 2019 Updated QBraydio 4No ratings yet
- Test For AcidsDocument2 pagesTest For AcidsBeeNo ratings yet
- ATAR Chemistry Year 12 Asc 2017 Sols (WA)Document12 pagesATAR Chemistry Year 12 Asc 2017 Sols (WA)Raghav GanaNo ratings yet
- ATAR Chemistry Year 12 Asc 2017 (WA)Document12 pagesATAR Chemistry Year 12 Asc 2017 (WA)Raghav GanaNo ratings yet
- Nahco O ! Na: + H + Hco (100%ionization)Document5 pagesNahco O ! Na: + H + Hco (100%ionization)Nadya CRNo ratings yet
- Acids QuizDocument462 pagesAcids Quizwondimu0% (1)
- Titrations PH pOH354Document11 pagesTitrations PH pOH354estellasr00No ratings yet
- Additional Aspects of Aqueous Equilibria (AP MC)Document4 pagesAdditional Aspects of Aqueous Equilibria (AP MC)Sumolmal SrisukriNo ratings yet
- Prep For FT Hydrolysis and BufferDocument10 pagesPrep For FT Hydrolysis and BufferMutiara DhitaNo ratings yet
- PYQ 7equilibria-A2Document41 pagesPYQ 7equilibria-A2Deviantus1230% (2)
- Chem 1212 Exam KeyDocument6 pagesChem 1212 Exam KeyChris HeNo ratings yet
- TEST 02 September 2ndDocument10 pagesTEST 02 September 2ndNayyir Mumtasir Rahman 2323059047No ratings yet
- Acids & Bases (AP MC)Document7 pagesAcids & Bases (AP MC)rbarman1No ratings yet
- AP Acids and Bases Practice Problems 2020Document13 pagesAP Acids and Bases Practice Problems 2020Aindrila KaziNo ratings yet
- Acids and Bases StudentDocument24 pagesAcids and Bases StudentVictor BritoNo ratings yet
- Review Unit 10 Test CHP 17Document13 pagesReview Unit 10 Test CHP 17TechnoKittyKittyNo ratings yet
- Acids Bases QP - 2Document48 pagesAcids Bases QP - 2wnh8wyq97gNo ratings yet
- Worksheet 5 (Acids-Bases III) With AnswersDocument2 pagesWorksheet 5 (Acids-Bases III) With AnswersDelilah StephenieNo ratings yet
- Chapter 18 Multiple-choice questions - 複本Document16 pagesChapter 18 Multiple-choice questions - 複本connieNo ratings yet
- Acids, BAIS AND SALTS QDocument10 pagesAcids, BAIS AND SALTS Qexan14431No ratings yet
- 2023 H2 Chemical Equilibria Tutorial (QP)Document15 pages2023 H2 Chemical Equilibria Tutorial (QP)nivind88No ratings yet
- Tutorial 6 (Acid-Base Equilibria and Buffers)Document5 pagesTutorial 6 (Acid-Base Equilibria and Buffers)Ahmed ZakyNo ratings yet
- ANSWERS To Chapters 9 - 11 Homework Supplement-2Document12 pagesANSWERS To Chapters 9 - 11 Homework Supplement-2JacobNo ratings yet
- HL Topic 8 Acids and Bases - 10 September 2020Document13 pagesHL Topic 8 Acids and Bases - 10 September 2020ellie du123No ratings yet
- Tutorial 10Document3 pagesTutorial 10nrinsyirah1806No ratings yet
- HCH111 Quiz 2, Answers, 2019Document2 pagesHCH111 Quiz 2, Answers, 2019Bonita NengweNo ratings yet
- 1BAcid Base Worksheet1Document4 pages1BAcid Base Worksheet1Pramudith LiyanageNo ratings yet
- Acids and Bases MCDocument70 pagesAcids and Bases MCRasel IslamNo ratings yet
- 1970-1978 Acid BaseDocument5 pages1970-1978 Acid BaseJanine McLaughlinNo ratings yet
- Treatment For Multiple Chem EquilDocument157 pagesTreatment For Multiple Chem EquilEndah SuarsihNo ratings yet
- Lecture 6 Acids and Bases v2Document42 pagesLecture 6 Acids and Bases v2Yahmeela SernaNo ratings yet
- Equilibrium Practice AnswersDocument4 pagesEquilibrium Practice AnswersakshayddsbNo ratings yet
- Kesetimbangan LarutanDocument123 pagesKesetimbangan LarutanFirda SafitriNo ratings yet
- Class Test 1: Section A (Multiple-Choice Questions)Document10 pagesClass Test 1: Section A (Multiple-Choice Questions)Kgaugelo TraciaNo ratings yet
- CHM221 AssignmentDocument1 pageCHM221 AssignmentGlory UsoroNo ratings yet
- Practice Test Acids BasesDocument4 pagesPractice Test Acids Basesdemetri lanezNo ratings yet
- IB Chemistry - SL Topic 8 Questions 1Document15 pagesIB Chemistry - SL Topic 8 Questions 1Yoshua YanottamaNo ratings yet
- YT Acids and Bases SLDocument14 pagesYT Acids and Bases SLJulie HongNo ratings yet
- Le Chatelier's Principle - Chromate Dichromate C12!4!07Document7 pagesLe Chatelier's Principle - Chromate Dichromate C12!4!07Joe Marie VelasquezNo ratings yet
- 1979Document3 pages1979bobothebioguyNo ratings yet
- A Level Chemistry Paper 2 Exam 34Document5 pagesA Level Chemistry Paper 2 Exam 34Anthony AndyNo ratings yet
- 9.1 Kesetimbangan LarutanDocument136 pages9.1 Kesetimbangan Larutanlyla novitaNo ratings yet
- 1 - Acids & Bases Work SheetDocument4 pages1 - Acids & Bases Work SheetYogy YNo ratings yet
- Acids, Bases, and BuffersDocument8 pagesAcids, Bases, and BuffersPeshala NishadiNo ratings yet
- Chapter 15Document12 pagesChapter 15IsisahNo ratings yet
- Acid - Base Free Response Questions Ver 1 Partial AnswersDocument5 pagesAcid - Base Free Response Questions Ver 1 Partial AnswersPrime JackNo ratings yet
- Multiple Choice: CH142 Sample Exam 2 QuestionsDocument12 pagesMultiple Choice: CH142 Sample Exam 2 QuestionsRiky GunawanNo ratings yet
- Exercise: HCL + H O (Aq) + CL (Aq)Document3 pagesExercise: HCL + H O (Aq) + CL (Aq)baskieNo ratings yet
- s6 Chemistry Pp2Document5 pagess6 Chemistry Pp2ANYWAR SIMONNo ratings yet
- Form 3 The Mole Formulae and Chemical Equestionsuations Questions Teacher - Co .KeDocument5 pagesForm 3 The Mole Formulae and Chemical Equestionsuations Questions Teacher - Co .Kewanjirunjoroge379No ratings yet
- Calculations WBDocument13 pagesCalculations WBEstella BonananNo ratings yet
- Practice FRQDocument4 pagesPractice FRQKrystal LiNo ratings yet
- UntitledDocument2 pagesUntitledjillNo ratings yet
- H2 Equilibrium and Ideal GasDocument9 pagesH2 Equilibrium and Ideal GaskitoniumNo ratings yet
- Equilibrium Hsslive AnilDocument3 pagesEquilibrium Hsslive AnilDhana AryalNo ratings yet
- ATAR Chemistry Year 12 Asc 2018 Sols (WA)Document10 pagesATAR Chemistry Year 12 Asc 2018 Sols (WA)Raghav GanaNo ratings yet
- Quick Check 1 and 2Document2 pagesQuick Check 1 and 2Канат ТютеновNo ratings yet
- Station Review AnswersDocument5 pagesStation Review AnswersКанат ТютеновNo ratings yet
- Two Points AnswersDocument2 pagesTwo Points AnswersКанат ТютеновNo ratings yet
- Chapter 7 FULL PresentationDocument134 pagesChapter 7 FULL PresentationКанат ТютеновNo ratings yet
- IMF NotesDocument23 pagesIMF NotesКанат ТютеновNo ratings yet
- IMF WorksheetDocument3 pagesIMF WorksheetКанат ТютеновNo ratings yet
- Quick Check 1Document1 pageQuick Check 1Канат ТютеновNo ratings yet
- Heating CurveDocument1 pageHeating CurveКанат ТютеновNo ratings yet
- Hess S Law NotesDocument2 pagesHess S Law NotesКанат ТютеновNo ratings yet
- Le Chateliers Principle Practice 2Document2 pagesLe Chateliers Principle Practice 2Канат ТютеновNo ratings yet
- Introduction To EnergyDocument2 pagesIntroduction To EnergyКанат ТютеновNo ratings yet
- End Point Calculations AnswersDocument2 pagesEnd Point Calculations AnswersКанат ТютеновNo ratings yet
- Energies of Solution FormationDocument2 pagesEnergies of Solution FormationКанат ТютеновNo ratings yet
- IMF TrendsDocument1 pageIMF TrendsКанат ТютеновNo ratings yet
- 5 RadiationDocument8 pages5 RadiationКанат ТютеновNo ratings yet
- Chapter 13 Study QuestionsDocument2 pagesChapter 13 Study QuestionsКанат ТютеновNo ratings yet
- Chapter 7 Quick Check 3Document1 pageChapter 7 Quick Check 3Канат ТютеновNo ratings yet
- End Point CalculationsDocument2 pagesEnd Point CalculationsКанат ТютеновNo ratings yet
- AP PPT CH 11Document68 pagesAP PPT CH 11Канат ТютеновNo ratings yet
- Ch14 Quick Check 3Document1 pageCh14 Quick Check 3Канат ТютеновNo ratings yet
- AP PPT CH 9 AP OnlyDocument42 pagesAP PPT CH 9 AP OnlyКанат ТютеновNo ratings yet
- Ch14 PH CalculationsDocument1 pageCh14 PH CalculationsКанат ТютеновNo ratings yet
- AP PPT CH 2 AP OnlyDocument26 pagesAP PPT CH 2 AP OnlyКанат ТютеновNo ratings yet