Professional Documents
Culture Documents
Rounding Negash
Uploaded by
kaldamtewOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Rounding Negash
Uploaded by
kaldamtewCopyright:
Available Formats
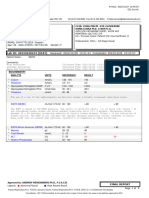
IDT Rounding Worksheet
Negash, Menberish DOB: 12/30/1951 | MPI: 2204789
Filters
Include Section: Active Allergies; Active Dialysis Access(es); Problem List; Labs; Treatment Info; Visit Info; Peritoneal Equilibration Test(s) (PET); Active Treatment Order(s);
Active Medication(s); Active Protocol Order(s); Hospitalization Event(s) (Within the last 31 days); Microbiology Culture, Antibiotic
Primary Nephrologist: Upadhyay, Ashish MD Actual DaVita Start Date: 01/23/2020 Advance Care Plan Status: Full Code
Renal Function Status: ESRD Regular Chronic Dialysis Began: 03/28/2020 Latest PHQ-9 Score: 0,01/24/2023
Diabetic Status: Diabetes Mellitus - Type 2 Treatment Schedule: M W F; Shift 2 (03035) Height (BMI): 63.00in / 5ft 3.00in / 160.02cm (28.1)
Modality Status: In-Center Hemodialysis (ICHD) Treatment Duration: 03:33 (213 mins) Target Weight (TW): 72.0 kg (158.7 lbs)
* Last Used Access(es)
ACTIVE ALLERGIES ACTIVE DIALYSIS ACCESS(ES) SITE DATE PLACED
No Known Allergies *AV Fistula Upper Arm (Left) 06/24/2020
PROBLEM LIST
No Data Available.
🏥 Outside labs may use a different unit of measure | Underlined: Out of Range
LABS
ANEMIA JAN 2024 DEC 2023 NOV 2023 ADEQUACY JAN 2024 DEC 2023 NOV 2023
HEMOGLOBIN (g/dL) 11.5 (12/27) STDKT/V (DIAL) N/A (01/03) N/A (12/06) N/A (11/01)
Drawn (01/08) 12.3 (12/20) 12 (11/21) SPKT/V 1.92 (01/03) 2.14 (12/06) 2.57 (11/01)
11.8 (01/03) 11.8 (12/06) 11.9 (11/01) STDKT/V TOTAL N/A (01/03) N/A (12/06) N/A (11/01)
IRON SATURATION 25 (01/03) 30 (12/06) BUN (mg/dL) 52 (01/03) 43 (12/06) 62 (11/01)
(%)
BUN - POST (mg/dL) 10 (01/03) 7 (12/06) 8 (11/01)
FERRITIN (ng/mL) 929 (01/03) 1061 (12/06)
URR% (%) 81 (01/03) 84 (12/06) 87 (11/01)
IRON (ug/dL) 65 (01/03) 77 (12/06)
CREATININE (mg/dL) 8.1 (01/03) 7.3 (12/06) 7.73 (11/01)
MCV (fL) 93.2 (01/03) 93.7 (12/06) 94 (11/01)
NUTRITION JAN 2024 DEC 2023 NOV 2023
TIBC (ug/dL) 261 (01/03) 260 (12/06)
ALBUMIN (g/dL) 4.1 (01/03) 4.3 (12/06) 4.2 (11/01)
WBC (x 10'3 cells/uL) 8.9 (01/03) 8.6 (12/06) 8.4 (11/01)
NPCR (G/KG/D) 0.88 (01/03) 1.01 (12/06) 1.54 (11/01)
MBD JAN 2024 DEC 2023 NOV 2023
POTASSIUM (mEq/L) 5.1 (11/21)
CA CORRECTED 9.4 (01/03) 9.7 (12/06) 9.4 (11/01)
(mg/dL) 5.7 (12/20) 5.1 (11/08)
CALCIUM (mg/dL) 9.4 (01/03) 9.7 (12/06) 9.4 (11/01) 5.8 (01/03) 4.9 (12/06) 5.4 (11/01)
PHOSPHORUS 3.8 (01/03) 4.1 (12/06) 4.9 (11/01) SODIUM (mEq/L) 137 (01/03) 140 (12/06) 137 (11/01)
(mg/dL)
CO2 (mEq/L) 24 (01/03) 27 (12/06) 27 (11/01)
PTH-INTACT (pg/mL) 230 (01/03) 154 (12/06) 327 (11/01)
TREATMENT INFO (03035) BOSTON DIALYSIS
TREATMENT DATE 01/08/24 01/05/24 01/03/24 12/31/23 12/29/23 12/27/23 12/24/23 12/22/23 12/20/23 12/18/23 12/15/23 12/13/23 12/11/23
TREATMENT ORDER ICHD ICHD ICHD ICHD ICHD ICHD ICHD ICHD ICHD ICHD ICHD ICHD ICHD
TYPE
FACILITY (03035) (03035) (03035) (03035) (03035) (03035) (03035) (03035) (03035) (03035) (03035) (03035) (03035)
Pre Weight (kg) 74 74 75.1 74 75.5 73.5 74.1 75 74.4 75.3 74.1 74 75.4
Post Weight (kg) 72 72 72.7 72.8 72 71.6 72.5 73.4 72.7 73 72 72.5 73.4
Target Weight (kg) 72 72 72 72 72 72 72 72 72 72 72 72 72
IDWG (kg) 2.0 1.3 2.3 2.0 3.9 1.0 0.7 2.3 1.4 3.3 1.6 0.6 3.3
DISCLAIMER: Printing information from CWOW should be limited due to regulatory compliance requirements. When printing is desired, it should only be for valid operational, clinical or treatment purposes and
consistent with DaVita policies and procedures. One must promptly shred or properly dispose the printed information when the purpose for which it was printed is completed. Failure to adhere to these requirements
may result in disciplinary action, up to and including termination of employment with DaVita.
BOSTON DIALYSIS (03035)
Page 1 of 4 | ver. R19.0.0.0-CWOW-104424 660 HARRISON AVE, BOSTON, MA 02118-2304 Printed
IDT_v.1.0.3_R19 © 2024 DaVita Inc. 01/08/2024 15:13 EST
IDT Rounding Worksheet
Negash, Menberish DOB: 12/30/1951 | MPI: 2204789
TREATMENT INFO (03035) BOSTON DIALYSIS
TREATMENT DATE 01/08/24 01/05/24 01/03/24 12/31/23 12/29/23 12/27/23 12/24/23 12/22/23 12/20/23 12/18/23 12/15/23 12/13/23 12/11/23
TREATMENT ORDER ICHD ICHD ICHD ICHD ICHD ICHD ICHD ICHD ICHD ICHD ICHD ICHD ICHD
TYPE
FACILITY (03035) (03035) (03035) (03035) (03035) (03035) (03035) (03035) (03035) (03035) (03035) (03035) (03035)
IDWG % of TW 2.8% 1.8% 3.2% 2.8% 5.4% 1.4% 1% 3.2% 1.9% 4.6% 2.2% 0.8% 4.6%
Machine Set To
2400 2600 2500 2000 3000 2100 2000 2500 2000 2500 2000 2200 2000
Remove (mL)
Total Volume
2408 1962 2260 1500 2862 2100 2000 2504 2006 2500 2005 2199 2005
Removed (mL)
Ultrafiltration Rate
7.8 7.6 9.3 4.7 14.6 7.9 6.6 6.1 6.4 9.0 8.3 5.9 7.7
(ml/kg/hr)
Pre BP Sit (mmHg) 109/51 113/55 135/66 103/54 122/62 123/56 109/64 131/63 114/57 107/53 126/61 101/72 124/64
Post BP Sit (mmHg) 121/53 118/77 131/96 106/55 126/62 124/64 135/72 142/70 132/85 118/57 126/67 136/78 122/73
Lowest BP (mmHg) 85/22 54/31 97/58 53/24 91/61 114/58 85/47 97/70 100/65 107/53 103/56 87/47 106/46
Prescribed Tx Time
3:30 3:30 3:30 3:30 3:30 3:30 3:30 3:30 3:30 3:30 3:30 3:30 3:30
(HH:MM)
Actual TX Time
03:33 03:40 03:32 03:29 03:20 03:22 03:20 03:33 03:39 03:30 03:31 03:30 03:32
(HH:MM)
NOTE: 5% of Current Target Weight is 3.6 kg
VISIT INFO
No Data Available.
PERITONEAL EQUILIBRATION TEST(S) (PET)
No Data Available.
ACTIVE TREATMENT ORDER(S)
IN-CENTER HEMODIALYSIS TREATMENT - [BOSTON DIALYSIS] (03035) Order ID: 241009082
Start Date 11/17/2023 Sequential UF N Sodium Modeling N
End Date - Max UF Rate 13 mL/kg/hr Base Sodium 138 mEq/L
Justification What is the justification?
End Stage Renal Disease Dialyzer Fresenius, Optiflux, UF Profiling Y (Every Treatment)
(ESRD) (N18.6,Z99.2); F160NR, 1025 Profile(s) #14
Frequency/TX Time Three times a week (210 Dialysate Bath FMC, ACID - LIQUID,
mins) 2K-2.5Ca, 55 GAL Provider Upadhyay, Ashish MD
Target Weight 72 kg DRUM Collaborating Physician -
Blood Flow Rate 450 mL/min Bicarbonate 38 mEq/L
Dialysate Flow Rate 800 mL/min Dialysate Temperature 36°C
Concurrent Access No
Arterial Access AV Fistula (Upper Arm
(Left))
Arterial Needle NIPRO, TULIP, 15G x 1",
SHARP , TWIN
Venous Access AV Fistula (Upper Arm
(Left))
Venous Needle NIPRO, TULIP, 15G x 1",
SHARP , TWIN
DISCLAIMER: Printing information from CWOW should be limited due to regulatory compliance requirements. When printing is desired, it should only be for valid operational, clinical or treatment purposes and
consistent with DaVita policies and procedures. One must promptly shred or properly dispose the printed information when the purpose for which it was printed is completed. Failure to adhere to these requirements
may result in disciplinary action, up to and including termination of employment with DaVita.
BOSTON DIALYSIS (03035)
Page 2 of 4 | ver. R19.0.0.0-CWOW-104424 660 HARRISON AVE, BOSTON, MA 02118-2304 Printed
IDT_v.1.0.3_R19 © 2024 DaVita Inc. 01/08/2024 15:13 EST
IDT Rounding Worksheet
Negash, Menberish DOB: 12/30/1951 | MPI: 2204789
ACTIVE TREATMENT ORDER(S)
SEQUENTIAL TREATMENT - [BOSTON DIALYSIS] (03035) Order ID: 1900816410
Start Date 11/05/2022 Sequential UF N Sodium Modeling N
End Date - Max UF Rate 13 mL/kg/hr Base Sodium 138 mEq/L
Justification What is the justification?
End Stage Renal Disease Dialyzer Gambro, Revaclear, Auto Flow No
(ESRD) (N18.6,Z99.2); 300, 1218 UF Profiling Y (Every Treatment)
UF Justification Fluid Bicarbonate 38 mEq/L Profile(s) #19
overload due to UF Dialysate Temperature 36.5°C
intolerance (E87.79); Provider Bhatia, Jasvinder S. MD
Fluid Overload UF Collaborating Physician -
Documentation
Intradialytic
hypotension;
Frequency/TX Time One time only (120
mins)
Target Weight 72 kg
Blood Flow Rate 450 mL/min
Dialysate Flow Rate 800 mL/min
Concurrent Access No
Arterial Access AV Fistula (Upper Arm
(Left))
Arterial Needle NIPRO, TULIP, 15G x 1",
SHARP , TWIN
Venous Access AV Fistula (Upper Arm
(Left))
Venous Needle NIPRO, TULIP, 15G x 1",
SHARP , TWIN
*Per Protocol Order
ACTIVE MEDICATION(S) Bundle Type: ALL
IN-CENTER MEDICATION DOSE/ROUTE/FREQUENCY FACILITY PROVIDER START DATE
*Mircera 75 Microgram, Intravenous, ESA dosing, every four BOSTON DIALYSIS(03035) Bhatia, Jasvinder, 01/04/2024
weeks S.MD
*Calcitriol 2.25 Microgram, Oral, Three times a week BOSTON DIALYSIS(03035) Bhatia, Jasvinder, 12/12/2023
S.MD
Heparin Pork, Hourly Dose 500 Units/Hr, Intravenous, Every Dialysis Treatment | BOSTON DIALYSIS(03035) Bhatia, Jasvinder, 09/18/2022
Stop Time 60 minutes S.MD
Heparin Pork, Loading Dose 1,000 Units, Intravenous, Every Dialysis Treatment BOSTON DIALYSIS(03035) Bhatia, Jasvinder, 09/18/2022
S.MD
HOME MEDICATION DOSE/ROUTE/FREQUENCY PROVIDER START DATE
Ammonium Lactate 1 Application, External, As needed 06/27/2022
Daily-Vite 1 Tablet, Oral, One time a day 06/27/2022
Lanolin Alcohol 1 -, Not Applicable, Two times a day 06/27/2022
Provider Instructions: apply BID to affected areas.
Lantus 8 Unit, Subcutaneous, Every evening 06/27/2022
Pantoprazole Sodium 20 Milligram, Oral, One time a day 06/27/2022
valACYclovir HCl 500 Milligram, Oral, One time a day 06/27/2022
Acetaminophen 500 Milligram, Oral, Every six hours 12/04/2021
Aspirin 81 Milligram, Oral, One time a day 12/04/2021
Carvedilol 12.50 Milligram, Oral, Two times a day 12/04/2021
Loratadine 10 Milligram, Oral, One time a day 12/04/2021
NIFEdipine 120 Milligram, Oral, One time a day 12/04/2021
DISCLAIMER: Printing information from CWOW should be limited due to regulatory compliance requirements. When printing is desired, it should only be for valid operational, clinical or treatment purposes and
consistent with DaVita policies and procedures. One must promptly shred or properly dispose the printed information when the purpose for which it was printed is completed. Failure to adhere to these requirements
may result in disciplinary action, up to and including termination of employment with DaVita.
BOSTON DIALYSIS (03035)
Page 3 of 4 | ver. R19.0.0.0-CWOW-104424 660 HARRISON AVE, BOSTON, MA 02118-2304 Printed
IDT_v.1.0.3_R19 © 2024 DaVita Inc. 01/08/2024 15:13 EST
IDT Rounding Worksheet
Negash, Menberish DOB: 12/30/1951 | MPI: 2204789
*Per Protocol Order
ACTIVE MEDICATION(S) Bundle Type: ALL
HOME MEDICATION DOSE/ROUTE/FREQUENCY PROVIDER START DATE
Nateglinide 60 Milligram, Oral, With meals 12/04/2021
Provider Instructions: TID
Sevelamer Carbonate 1,600 Milligram, Oral, With meals 12/04/2021
Provider Instructions: 2--800mg tabs TID
Tradjenta 1 Tablet, Oral, One time a day 12/04/2021
Provider Instructions: 5mg
Simvastatin 10 Milligram, Oral, One time a day 01/29/2020
ACTIVE PROTOCOL ORDERS START DATE LAST REVIEW DATE
ICHD Oral Calcitriol Given In-center (Rev 2.0) 04/26/2023 12/11/2023
ICHD SHAPE IV Mircera rev 4.2 04/11/2023 01/04/2024
ICHD Iron Works (IV Iron Sucrose) Protocol, Hold for Ferritin > 800, Revision 4.1 09/18/2022 01/04/2024
Oral Nutrition Supplement (ONS) Protocol (rev 3.0) 09/18/2022 12/11/2023
MBD Lab Monitoring Protocol (Revision 1.0) 09/18/2022
Hepatitis C Surveillance Twice a Year Protocol Rev 3.1 09/18/2022
HOSPITALIZATION EVENT(S) Within the last 31 Days
No Data Available.
🏥 Outside Labs may use a different unit of measure
MICROBIOLOGY CULTURE
No Data Available.
ANTIBIOTIC
No Data Available.
The purpose of this worksheet is for the interdisciplinary team to review clinical information and discuss observations while rounding with providers. If notes are handwritten on this
worksheet it must be retained in the medical record.
Name
Signature Date
DISCLAIMER: Printing information from CWOW should be limited due to regulatory compliance requirements. When printing is desired, it should only be for valid operational, clinical or treatment purposes and
consistent with DaVita policies and procedures. One must promptly shred or properly dispose the printed information when the purpose for which it was printed is completed. Failure to adhere to these requirements
may result in disciplinary action, up to and including termination of employment with DaVita.
BOSTON DIALYSIS (03035)
Page 4 of 4 | ver. R19.0.0.0-CWOW-104424 660 HARRISON AVE, BOSTON, MA 02118-2304 Printed
IDT_v.1.0.3_R19 © 2024 DaVita Inc. 01/08/2024 15:13 EST
You might also like
- Atlantic Cod: A Bio-EcologyFrom EverandAtlantic Cod: A Bio-EcologyGeorge A. RoseNo ratings yet
- Sow Form 2 2021 With Penjajaran NurulDocument4 pagesSow Form 2 2021 With Penjajaran NurulSharan Hans100% (5)
- Burden of Coronary Artery Disease As A Cause o - 2019 - Journal of The AmericanDocument3 pagesBurden of Coronary Artery Disease As A Cause o - 2019 - Journal of The AmericanSPN CDYNo ratings yet
- Date 02/dec/2021 02:03AM 01/dec/21 03:53AM 30/nov/21 04:27AM 29/nov/21 04:25AM 28/nov/21 04:21AM Unit Bio Ref IntervalDocument3 pagesDate 02/dec/2021 02:03AM 01/dec/21 03:53AM 30/nov/21 04:27AM 29/nov/21 04:25AM 28/nov/21 04:21AM Unit Bio Ref IntervalSaurabh PuriNo ratings yet
- February 9 Census ADMISSIONS February 5-6: General Data Working Diagnosis Laboratories Medications On Board NotesDocument8 pagesFebruary 9 Census ADMISSIONS February 5-6: General Data Working Diagnosis Laboratories Medications On Board NotesLuis PadillaNo ratings yet
- National Competition Case: ASHP Clinical Skills CompetitionDocument16 pagesNational Competition Case: ASHP Clinical Skills CompetitionHelmiNo ratings yet
- Max Lab ReportDocument4 pagesMax Lab ReportKallu PrasadNo ratings yet
- Form 2 Wsow 2023Document4 pagesForm 2 Wsow 2023thatchayinee jagathesanNo ratings yet
- Bedah Digestif 30 Agustus 2020Document1 pageBedah Digestif 30 Agustus 2020Hengky TanNo ratings yet
- NSPDocument3 pagesNSProbhendryxNo ratings yet
- Date 24/dec/2022 08:32AM 03/dec/22 09:33AM 26/nov/22 09:05AM Unit Bio Ref IntervalDocument11 pagesDate 24/dec/2022 08:32AM 03/dec/22 09:33AM 26/nov/22 09:05AM Unit Bio Ref IntervalAmit SinghNo ratings yet
- SVLT Lab Report DetailsDocument2 pagesSVLT Lab Report DetailsDaya RaniNo ratings yet
- Solar Cell Efficiency Tables (Version 58)Document11 pagesSolar Cell Efficiency Tables (Version 58)劉鋒No ratings yet
- Bharathi 1Document4 pagesBharathi 1Rekha ShriNo ratings yet
- Lyphochek Assayed Chemistry Control Levels 1 and 2: Revision Date 2024-03-12 Indicates Revised InformationDocument2 pagesLyphochek Assayed Chemistry Control Levels 1 and 2: Revision Date 2024-03-12 Indicates Revised InformationGaurav MauryaNo ratings yet
- Jurnal Injeksi PhenoDocument10 pagesJurnal Injeksi Phenoastriani oktaviaNo ratings yet
- Lyphochek Assayed Chemistry Control Levels 1 and 2: Tanggal Revisi 2023-04-06 Menunjukkan Informasi Yang DirevisiDocument8 pagesLyphochek Assayed Chemistry Control Levels 1 and 2: Tanggal Revisi 2023-04-06 Menunjukkan Informasi Yang Direvisiheru ramadhanNo ratings yet
- Chesmistry Trulab Pro Line LOT 89711 310-11-2024Document22 pagesChesmistry Trulab Pro Line LOT 89711 310-11-2024seksi sspk sarprasNo ratings yet
- Report OBDocument2 pagesReport OBShokimun Mega SamuderaNo ratings yet
- Report-January 2019: Hospital Infection Control (Hic)Document27 pagesReport-January 2019: Hospital Infection Control (Hic)Sureshkumar ManoharanNo ratings yet
- Laboratorios Ramirez: Analysis Report Patient Number Birthdate SexDocument1 pageLaboratorios Ramirez: Analysis Report Patient Number Birthdate SexFernando TorresNo ratings yet
- Sow Form 2 2021 With PenjajaranDocument4 pagesSow Form 2 2021 With PenjajaranCik Nonie RahimNo ratings yet
- Lelly Muridi Zham Zham - Rep - OBDocument4 pagesLelly Muridi Zham Zham - Rep - OBPutera BrahmansaNo ratings yet
- 8, Identitas Pasien SoapDocument10 pages8, Identitas Pasien SoapsunarsihNo ratings yet
- Lab ID: 20233620234 (STAT) Received: 2023/12/28 13:51:41 Completed: 2023/12/28 15:23:57Document5 pagesLab ID: 20233620234 (STAT) Received: 2023/12/28 13:51:41 Completed: 2023/12/28 15:23:57Liu HànNo ratings yet
- Haematology: Investigation Observed Value Unit Biological Reference IntervalDocument9 pagesHaematology: Investigation Observed Value Unit Biological Reference Intervalsunita hoskotiNo ratings yet
- Green Et Al-2019-Progress in Photovoltaics Research and ApplicationsDocument10 pagesGreen Et Al-2019-Progress in Photovoltaics Research and ApplicationsIrfanKhanNo ratings yet
- An Assessment of River Water Quality: The River Tavy Located in The South West of EnglandDocument13 pagesAn Assessment of River Water Quality: The River Tavy Located in The South West of Englandapi-588254617No ratings yet
- Genetic Aetiology of Glycaemic Traits: Approaches and InsightsDocument13 pagesGenetic Aetiology of Glycaemic Traits: Approaches and InsightsMila AnasantiNo ratings yet
- FCBB6Document6 pagesFCBB6sweett310No ratings yet
- Lyphochek Immunoassay Plus Control Levels 1, 2 and 3: Revision Date 2023-03-21 Indicates Revised InformationDocument2 pagesLyphochek Immunoassay Plus Control Levels 1, 2 and 3: Revision Date 2023-03-21 Indicates Revised InformationMizanur RahmanNo ratings yet
- Department of Haematology - : Mr. Nagaraja N 72yr 0Mth 3days Male Uhid Sin /LRN W/Bno/RefnoDocument1 pageDepartment of Haematology - : Mr. Nagaraja N 72yr 0Mth 3days Male Uhid Sin /LRN W/Bno/RefnoPrashanth NNo ratings yet
- Reagen Stok 2Document41 pagesReagen Stok 2Laboratorium RSUMMNo ratings yet
- Seery, Marilyn R.: Patient ReportDocument2 pagesSeery, Marilyn R.: Patient ReportMarilyn SeeryNo ratings yet
- Chemistry - I: TEST(s) Normal UNIT(s)Document1 pageChemistry - I: TEST(s) Normal UNIT(s)Syed Muhammad Zubair TariqNo ratings yet
- Mangalabai Kumbhar29Document5 pagesMangalabai Kumbhar29reddroppathologylab21No ratings yet
- Ham 2428683. 25215Document2 pagesHam 2428683. 25215ahmad saadNo ratings yet
- Rna Formulation For Immunoterapy Us10485884Document69 pagesRna Formulation For Immunoterapy Us10485884simulinneissau-4514No ratings yet
- ModifiedDocument1 pageModifiedDinil KannurNo ratings yet
- Lyphochek Assayed Chemistry Control Levels 1 and 2: MéthodeDocument1 pageLyphochek Assayed Chemistry Control Levels 1 and 2: MéthodeAnouar Nouioui100% (2)
- CBC - CPDocument1 pageCBC - CPsammy khanNo ratings yet
- Lembar Pengobatan: Jl. Dr. Abdul Rivai No.1, Bukittinggi, Sumatera Barat 26136Document9 pagesLembar Pengobatan: Jl. Dr. Abdul Rivai No.1, Bukittinggi, Sumatera Barat 26136Adisti PutriNo ratings yet
- (22.45) Mapping Aqsa 2 Minggu 14 - 02 - 2021Document8 pages(22.45) Mapping Aqsa 2 Minggu 14 - 02 - 2021ifanda80No ratings yet
- Biochemistry: Mrs - Durva Shukla 29 Years / Female Maithili Ghadge 2011713370 177801575942 26-Apr-2020 / 12:24 R E P O R TDocument7 pagesBiochemistry: Mrs - Durva Shukla 29 Years / Female Maithili Ghadge 2011713370 177801575942 26-Apr-2020 / 12:24 R E P O R TSachin AgarwalNo ratings yet
- Lyphochek Assayed Chemistry Control Levels 1 and 2: Date de Révision 2023-01-10 Indique Des Informations RéviséesDocument1 pageLyphochek Assayed Chemistry Control Levels 1 and 2: Date de Révision 2023-01-10 Indique Des Informations RéviséesAnouar NouiouiNo ratings yet
- 001 201373074 CB6 117 3 PDFDocument1 page001 201373074 CB6 117 3 PDFknowledge WorldNo ratings yet
- Solar Cell Efficiency Tables (Version 50)Document9 pagesSolar Cell Efficiency Tables (Version 50)Tvarit PatelNo ratings yet
- Us11285335Document14 pagesUs11285335Anonymous KiraNo ratings yet
- Thyrocare Processed atDocument1 pageThyrocare Processed atijunoamjadNo ratings yet
- Serial Laboratory Values Beginning Nine Months Prior To Present VisitDocument1 pageSerial Laboratory Values Beginning Nine Months Prior To Present VisitKath Dela CruzNo ratings yet
- Wellness 5 Test Panel ResultsDocument3 pagesWellness 5 Test Panel Resultssaipavani kummaraguntlaNo ratings yet
- Date 26/dec/2021 10:07AM Unit Bio Ref Interval: KFT Profile With Calcium, Uric Acid, SerumDocument3 pagesDate 26/dec/2021 10:07AM Unit Bio Ref Interval: KFT Profile With Calcium, Uric Acid, SerumhsclogicsNo ratings yet
- Amprahan HCU Senin Sore, 27 November 2023Document8 pagesAmprahan HCU Senin Sore, 27 November 2023alfaz zamzamiNo ratings yet
- Https WWW - Midas.com - NP Hospital Labreports Reports Reports Index 2079-N36700Document2 pagesHttps WWW - Midas.com - NP Hospital Labreports Reports Reports Index 2079-N36700ABhishek KishanNo ratings yet
- Haematology: Investigation Observed Value Unit Biological Reference IntervalDocument2 pagesHaematology: Investigation Observed Value Unit Biological Reference IntervalVivek RadhakrishnanNo ratings yet
- D5683 VE 1 Susy 3561Document2 pagesD5683 VE 1 Susy 3561melendezjmanuelNo ratings yet
- ReportDocument5 pagesReportRoy YadavNo ratings yet
- Blood TestDocument2 pagesBlood TestAnonymous RPgVLiT5MoNo ratings yet
- TEST(s) Normal UNIT(s)Document1 pageTEST(s) Normal UNIT(s)Ahad Siddique100% (1)
- United States Patent: Weir Jan - 30, 2018Document28 pagesUnited States Patent: Weir Jan - 30, 2018Manuel Rios GonzalesNo ratings yet
- Abstract Book Leish World Congress 5Document1,116 pagesAbstract Book Leish World Congress 5libremdNo ratings yet
- Timing Advance UnitDocument9 pagesTiming Advance UnitShohan Taylor50% (2)
- Precast Prestressed Concrete Horizontally Curved Bridge BeamsDocument42 pagesPrecast Prestressed Concrete Horizontally Curved Bridge BeamsEdward van Martino100% (1)
- Braden Bga06 Manual de PartesDocument24 pagesBraden Bga06 Manual de PartesMauricio Ariel H. OrellanaNo ratings yet
- Guide Isolators 2017Document180 pagesGuide Isolators 2017GhiloufiNo ratings yet
- Aft MPM Cafs 01Document2 pagesAft MPM Cafs 01Forum PompieriiNo ratings yet
- Sony dcr-sr190 sr200 sr290 sr300 Level2 Ver1.0 (ET)Document96 pagesSony dcr-sr190 sr200 sr290 sr300 Level2 Ver1.0 (ET)Dedu TelespanNo ratings yet
- Airports 14 EndDocument9 pagesAirports 14 EndMeedenken en Doen BVNo ratings yet
- Mobility Driven Network Slicing: An Enabler of On Demand Mobility Management For 5GDocument12 pagesMobility Driven Network Slicing: An Enabler of On Demand Mobility Management For 5GgirishryenniNo ratings yet
- Lecture 1Document46 pagesLecture 1Imtiyaaz MalickNo ratings yet
- IELTS Reading Skills & StrategiesDocument4 pagesIELTS Reading Skills & StrategiesThinn Thinn Oo100% (1)
- High Voltage Test of All Electrical EquipmentsDocument132 pagesHigh Voltage Test of All Electrical Equipmentsvurumuu100% (1)
- HFWB - 170224 - Floor StandingDocument2 pagesHFWB - 170224 - Floor StandingAkilaJosephNo ratings yet
- Drahmedsoil Mechanicsnoteschapter 2Document22 pagesDrahmedsoil Mechanicsnoteschapter 2kerby munarNo ratings yet
- Nutritional Impact On Performance in Student-Athletes - Reality AnDocument36 pagesNutritional Impact On Performance in Student-Athletes - Reality AnAbbygail WasilNo ratings yet
- Type Abz PDFDocument15 pagesType Abz PDFle khánhNo ratings yet
- Construction Quality Control Plan DraftDocument24 pagesConstruction Quality Control Plan Draftmozartjr22No ratings yet
- Weather and Climate-Cs VersionDocument19 pagesWeather and Climate-Cs VersionjtNo ratings yet
- BrevetDocument13 pagesBrevettyby77No ratings yet
- Mobile Management SystemDocument9 pagesMobile Management SystemNaga Sai Anitha NidumoluNo ratings yet
- Chapter 2 Linear MotionDocument12 pagesChapter 2 Linear MotionFatin AmirNo ratings yet
- Advanced Math Refresher SetDocument4 pagesAdvanced Math Refresher SetGracielle Nebres100% (1)
- Combined Cycle Power Plant - Summary StudyDocument3 pagesCombined Cycle Power Plant - Summary StudydndudcNo ratings yet
- HSE TerminologyDocument6 pagesHSE TerminologyZeshanNo ratings yet
- Escherichia Coli O157:H7 Issues and Ramifications: Executive SummaryDocument12 pagesEscherichia Coli O157:H7 Issues and Ramifications: Executive SummaryTinnysumardiNo ratings yet
- Vanishing TieDocument14 pagesVanishing TieIchwalsyah SyNo ratings yet
- TraderJoes CookbookDocument150 pagesTraderJoes Cookbookjennifer.farris12No ratings yet
- Suddenly One Summer by Fleur McDonald (Extract)Document15 pagesSuddenly One Summer by Fleur McDonald (Extract)Allen & UnwinNo ratings yet
- 2019 Iran PPRDocument10 pages2019 Iran PPRFareena FatimaNo ratings yet
- Activity Sheet - Radioactive DecayDocument5 pagesActivity Sheet - Radioactive DecayAkshat jhaNo ratings yet