Professional Documents
Culture Documents
Alpha5 M02296
Alpha5 M02296
Uploaded by
Paulo Dores0 ratings0% found this document useful (0 votes)
4 views1 pageOriginal Title
ALPHA5_M02296~
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
4 views1 pageAlpha5 M02296
Alpha5 M02296
Uploaded by
Paulo DoresCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 1
DECLARATION OF CONFORMITY
Manufactuer: Aloka Co., Ltd.
‘Address: 22-1 Mure 6-chome, Mitaka-shi, Tokyo 181-8622 Japan
European Representative: ALOKA GmbH
Address: Molisfeld 5,D-40670 Meerbusch,Germany
ee Ultrasound Diagnostic Equipment
Model Cade SSD-a5
Classification (MOD, Annex IX) Ta
We herewith declare that the above mentioned products including all its accessories meet the
provisions of the following EC Council Directives and Standards. All supporting documentations
are retained under the premises of the manufacturer and the notified body.
DIRECTIVES
General appicable directives:
Medical Device Directive: Council Directive 93/42/EEC of 14 June 1993 conceming
medical devices (MDD 93/42/EEC).
Standards: Harmonized Standards (published in the Office Journal of he European Commurities) applicable to
this product are
EN 60601~1(1990)/A1(1993)/A2(1995)
EN 60601-1~1(2001), EN 60601~1-2(2001)
EN 60601-1-4(1996)/A1(1999)
EN 60601-2-37(2001)/A1(2008), EN 980(2003)
‘Standards
EN ISO 14971(2000)/A\1(2003)
Noted body: TUV Product Service GmbH, Ridlerstrasse 65 D-80339 Munchen, Germany
Start of CE marking: SN: M02296
Place: Tokyo Works, Aloka Co., Ltd. , Ueda Factory, Aloka Co., Ltd,
— 26 Apr 2007
Siar AL bach,
Name ofissuer: Norikazu Okada
Position General Manager of Quality Assurance Department
ALOKA CO,LTD. L-166J-12-85-04
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5820)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (845)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Very Very Poor Ultrasound ImageDocument1 pageVery Very Poor Ultrasound ImagePaulo DoresNo ratings yet
- Olympus Compatibility ListDocument6 pagesOlympus Compatibility ListPaulo DoresNo ratings yet
- Aloka Co LTD Market Share AnalysisDocument5 pagesAloka Co LTD Market Share AnalysisPaulo DoresNo ratings yet
- eDMS Exam Answer 2002Document1 pageeDMS Exam Answer 2002Paulo DoresNo ratings yet
- SP-0900-V6.0-2.0 - Iec 2-37Document8 pagesSP-0900-V6.0-2.0 - Iec 2-37Paulo DoresNo ratings yet
- SMS 2.0 User Manual - 20160829 - V1.01Document37 pagesSMS 2.0 User Manual - 20160829 - V1.01Paulo DoresNo ratings yet
- SMS 2.0 User Manual - 20160829 - V1.02Document36 pagesSMS 2.0 User Manual - 20160829 - V1.02Paulo DoresNo ratings yet
- Addition NL1203Document1 pageAddition NL1203Paulo DoresNo ratings yet
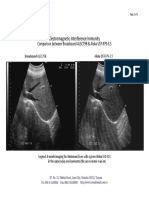
- 2008-04-25 - EII Comparison - Broadsound AL7L24B & Aloka UST-5524-7.5Document1 page2008-04-25 - EII Comparison - Broadsound AL7L24B & Aloka UST-5524-7.5Paulo DoresNo ratings yet
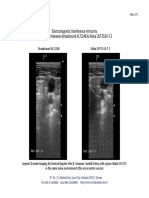
- 2009-05-02 - EII Comparison - Broadsound ME3C40B & Medison HC2-5Document1 page2009-05-02 - EII Comparison - Broadsound ME3C40B & Medison HC2-5Paulo DoresNo ratings yet
- 2009-01-10 - Source of Electromagnetic Interference - Servo MotorDocument1 page2009-01-10 - Source of Electromagnetic Interference - Servo MotorPaulo DoresNo ratings yet
- 2008-12-19 - EII Comparison - Broadsound AL3C79B & Aloka UST-979-3.5Document1 page2008-12-19 - EII Comparison - Broadsound AL3C79B & Aloka UST-979-3.5Paulo DoresNo ratings yet
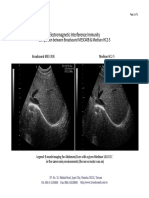
- 2008-12-13 - EII Comparison - Broadsound HTC314G & Hitachi EUP-C314GDocument1 page2008-12-13 - EII Comparison - Broadsound HTC314G & Hitachi EUP-C314GPaulo DoresNo ratings yet
- Ambisea - Electrocardigrafo - 1Ch - 3Ch - Folha A4Document1 pageAmbisea - Electrocardigrafo - 1Ch - 3Ch - Folha A4Paulo DoresNo ratings yet
- Ambisea - Electrocardigrafo - 1Ch - 3Ch - Folha A4.odgDocument1 pageAmbisea - Electrocardigrafo - 1Ch - 3Ch - Folha A4.odgPaulo DoresNo ratings yet
- Technology Corp., LTD: AV-7001V/7002V Veterinary Handheld Pulse OximeterDocument3 pagesTechnology Corp., LTD: AV-7001V/7002V Veterinary Handheld Pulse OximeterPaulo DoresNo ratings yet
- AMBIVisionPriceList200703Confidential ScannersDocument1 pageAMBIVisionPriceList200703Confidential ScannersPaulo DoresNo ratings yet