Professional Documents
Culture Documents
Aseptic Technique Biotech Protocol 2017
Uploaded by
babobusikOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Aseptic Technique Biotech Protocol 2017
Uploaded by
babobusikCopyright:
Available Formats
Aseptic Technique and Cell Culturing Protocol (2017)
This protocol describes basic procedures for aseptic technique for cell culture in biotechnology. This protocol
should be used along with pages 65-66 in your textbook. One basic concern for successful aseptic technique is
personal hygiene. The human skin harbors a naturally occurring and vigorous population of bacterial and fungal
inhabitants that shed microscopically and ubiquitously. Most unfortunately for cell culture work, cell culture
media and incubation conditions provide ideal growth environments for these potential microbial contaminants.
This procedure outlines steps to prevent introduction of human skin flora during aseptic culture manipulations.
Every item that comes into contact with a culture must be sterile. This includes direct contact (e.g., a pipet used
to transfer cells) as well as indirect contact (e.g., flasks or containers used to temporarily hold a sterile reagent
prior to aliquoting the solution into sterile media). In the biotechnology pathway we will mostly use equipment
that will need to be sterilized using the techniques of this protocol.
Materials: Antibacterial soap, 70% isopropanol, 91% isopropanol, clean laboratory coats or gowns, non-latex
surgical gloves, clean and quiet work area, Bunsen burners
Take personal precautions:
1. Just prior to aseptic manipulations, tie long hair back behind head. Vigorously scrub hands and arms at least 2
min with an antibacterial soap. Superficial lathering is more prone to loosening than removing flaking skin and
microbial contaminants. Loosely adhering skin flora easily dislodges and can fall into sterile containers.
2. Gown appropriately. For nonhazardous sterile-fill applications, wear clean laboratory coats and latex gloves.
Safety glasses should be worn when manipulating biological agents.
3. Frequently disinfect gloved hands with 70% isopropanol while doing aseptic work. Although the gloves may
initially have been sterile when first worn, they will no doubt have contacted many non-sterile items while in
use. Wash hands after removing gloves.
Prepare and maintain the work area:
4. Perform all aseptic work in a clean work space, free from contaminating air currents and drafts. For optimal
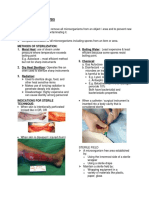
environmental control use a Bunsen burner to maintain a constant upward air current (figure 1). Never leave the
burner unattended when using this technique. One student must always be present, if not, turn the burner off.
5. Clear the work space of all items extraneous to the aseptic operation being performed.
6. Wipe down the work surface before and after use with 70% isopropanol or other appropriate disinfectant.
7. Wherever feasible, wipe down items with disinfectant as they are introduced into the clean work space.
Arrange necessary items in the work space in a logical pattern from clean to dirty to avoid passing contaminated
material (e.g., a pipet used to transfer cultures) over clean items (e.g., flasks of sterile media).
8. When the aseptic task has been completed, promptly remove any larger contaminated items or other material
meant for disposal (e.g., old culture material, spent media, waste containers) from the work space and place in
designated bags or pans for autoclaving. Disinfect the work space as in step 6.
Flame sterilize the opening of a vessel:
9. For a right-handed person, hold the vessel in the left hand at ∼45° angle (or as much as possible without
spilling contents; figure 2) and gently remove its closure. Do not permit any part of the closure that directly
comes in contact with the contents of the vessel to touch any contaminating object (e.g., hands or work bench).
Ideally, and with practice, one should be able to hold the closure in the crook of the little finger of the right hand
while still being able to manipulate an inoculating loop or pipettor with the other fingers of the hand. Holding
the vessel off vertical position while opening will prevent any airborne particulates from entering the container.
10. Slowly pass the opening of the vessel over the top of (rather than through) a Bunsen burner flame to burn
off any contaminating matter. Be careful when flaming containers of infectious material. Any liquid lodged in
the threads of a screw cap container will spatter as it is heated. Aerosols thus formed may actually disseminate
entrapped biological agents before the heat of the flame is hot enough to inactivate them.
11. While still holding the vessel at a slant, use a sterile pipet and pipettor to slowly add or remove aliquots to
avoid aerosol formation.
12. Flame-sterilize again as in step 10, allow the container to cool slightly, and carefully recap the vessel.
Flame sterilize small hand instruments:
13. Dip critical areas of the instrument (i.e., those that come into contact with the material) in 91% isopropanol.
Make certain that the alcohol is in a container heavy enough to support the instrument without tipping over.
CAUTION: 91% isopropanol is flammable; keep the container at a safe distance from any open flame.
14. Remove the instrument from the alcohol, being careful not to touch the disinfected parts of the instrument.
Allow excess ethanol to drain off into the container.
15. Pass the alcohol-treated part of the instrument through the flame of a Bunsen burner and allow residual
alcohol to burn off.
16. Do not let the sterilized portion of the instrument contact any nonsterile material before use. Let the heated
part of the instrument cool for ∼10 sec before use.
Flame sterilize inoculating loops and needles:
17. Hold the inoculating wire by its handle and begin in the center of the wire to slowly heat the wire with the
flame of a Bunsen burner. Proceed back and forth across the wire’s full length until it glows orange.
18. While still holding the handle, allow the inoculating wire to cool back to room temperature (∼10 sec) before
attempting any transfer of material. If tranfers are made while the inoculating wire is hot, cells will be killed by
the hot wire, and aerosols created from spattering material can disperse contaminants throughout the workspace.
19. After the transfer is made, reheat the inoculating wire as in step 17 to destroy any remaining biological
material. Let cool to room temperature before putting aside for next use.
Figure 1 Figure 2
CONTINUE TO NEXT PAGE
Figure 1: Streaking an agar plate is done in
3-4 sections. The goal is that sections “D”
and “E” will yield isolated, individual
colonies. Flame loop between each
section.
Figure 3: Always label the bottom of
the petri before pouring media.
Figure 2: Streaking and inoculating a
slant.
Figure 4: Technique for streaking a plate
in three sections using an inoculating
loop.
You might also like
- Taking A History of DEPRESSIONDocument3 pagesTaking A History of DEPRESSIONPrarthana Thiagarajan100% (13)
- Scott Cunningham - Cunningham's Encyclopedia of Wicca in The KitchenDocument6 pagesScott Cunningham - Cunningham's Encyclopedia of Wicca in The KitchenPandora Storm0% (1)
- Color PsychologyDocument10 pagesColor PsychologyRemya100% (1)
- Full Report Wastewater Paling Latest BiaDocument44 pagesFull Report Wastewater Paling Latest BiaSyazwi HakimiNo ratings yet
- Techniques For Studying Bacteria and Fungi PDFDocument32 pagesTechniques For Studying Bacteria and Fungi PDFRoshineeNo ratings yet
- Sterile TechniqueDocument13 pagesSterile TechniqueMhelrose GermataNo ratings yet
- Aseptic Technique and Pure CultureDocument42 pagesAseptic Technique and Pure CultureRachel Ng0% (1)
- HEM SOP Biological Safety v1Document8 pagesHEM SOP Biological Safety v1Kaoru AmaneNo ratings yet
- LAB 1 Aseptic TechniqueDocument4 pagesLAB 1 Aseptic TechniqueSyazwani Salleh100% (1)
- 3 Surgical Asepsis PDFDocument32 pages3 Surgical Asepsis PDFLimYiNo ratings yet
- Psilocybin Mushrooms: A step-by-step guide to growing your own magic fungi at homeFrom EverandPsilocybin Mushrooms: A step-by-step guide to growing your own magic fungi at homeNo ratings yet
- Submitted To: A Project Synopsis On Stress Management of Indian Bank EmployeesDocument49 pagesSubmitted To: A Project Synopsis On Stress Management of Indian Bank Employeesranjeet guptaNo ratings yet
- Basic Cell Culture TechniquesDocument22 pagesBasic Cell Culture TechniquestapanagnihotriNo ratings yet
- Lab 6 Aseptic TechniqueDocument22 pagesLab 6 Aseptic TechniqueKrsna NaveraNo ratings yet
- LESSON 3 Aseptic TechniqueDocument5 pagesLESSON 3 Aseptic TechniqueKezia Shayen RagualNo ratings yet
- Lab Safety and Microbiology TechniquesDocument48 pagesLab Safety and Microbiology TechniquesRaja Craze75% (8)
- Aseptic Technique: Preventing Harmful Bacterial ContaminationDocument3 pagesAseptic Technique: Preventing Harmful Bacterial ContaminationMarlene Tubieros - Inducil57% (7)
- Visitor T30 User's Manual - Rev07 PDFDocument37 pagesVisitor T30 User's Manual - Rev07 PDFDoaà SsalamNo ratings yet
- BT 0312 - Animal Cell and Tissue Culture LaboratoryDocument47 pagesBT 0312 - Animal Cell and Tissue Culture LaboratoryammaraakhtarNo ratings yet
- TOG Form 092A - Incident Investigation ReportDocument4 pagesTOG Form 092A - Incident Investigation ReportlinkencielNo ratings yet
- Subculturing TechniquesDocument20 pagesSubculturing TechniquesAgrobacterium Tumefaciens100% (3)
- Micro PrefinalsDocument24 pagesMicro PrefinalsMary Vinneizia CelecioNo ratings yet
- Biol1400 2 and 3Document12 pagesBiol1400 2 and 3Richelle Mae B. ButialNo ratings yet
- Aseptic Technique For Cell Culture: UNIT 1.3Document10 pagesAseptic Technique For Cell Culture: UNIT 1.3Bere CerecytaaNo ratings yet
- Streak Plate, Picking ColoniesDocument22 pagesStreak Plate, Picking ColoniesNorjetalexis Maningo CabreraNo ratings yet
- How To Do A Bacterial Streak Plate: Ethan FlintDocument5 pagesHow To Do A Bacterial Streak Plate: Ethan FlintEthan FlintNo ratings yet
- Biological Techniques Procedures and Methods - Asceptic Technique1Document39 pagesBiological Techniques Procedures and Methods - Asceptic Technique1Iphone. ColasticNo ratings yet
- Microbiology Lab Techniques and Safety ProceduresDocument30 pagesMicrobiology Lab Techniques and Safety ProceduresShahin Kauser ZiaudeenNo ratings yet
- BC4057 - Microbiology PracticalDocument18 pagesBC4057 - Microbiology PracticalLavinia MihaiNo ratings yet
- 112 Ex2Document3 pages112 Ex2Francis SullanoNo ratings yet
- Notes Based On 'Basic Practical Microbiology' © Society For General MicrobiologyDocument6 pagesNotes Based On 'Basic Practical Microbiology' © Society For General MicrobiologyTSha MeunierNo ratings yet
- Sterile TechniqueDocument1 pageSterile TechniqueBridget HaleyNo ratings yet
- Notes Based On 'Basic Practical Microbiology' © Society For General MicrobiologyDocument6 pagesNotes Based On 'Basic Practical Microbiology' © Society For General Microbiologygrace meunierNo ratings yet
- Microbiology TechniquesDocument18 pagesMicrobiology TechniquesArmil PequenaNo ratings yet
- Asepic HandlingDocument2 pagesAsepic HandlingRONAK LASHKARINo ratings yet
- Biological Spill Response ProceduresDocument3 pagesBiological Spill Response ProceduresWael OsmanNo ratings yet
- S.Y.B. Sc. SEM-IIDocument24 pagesS.Y.B. Sc. SEM-IIPallavi JadhavNo ratings yet
- Microbiology LAB 3 Sterilization and DisinfectionDocument2 pagesMicrobiology LAB 3 Sterilization and DisinfectionRaven GoseNo ratings yet
- Aseptic TransfersDocument6 pagesAseptic TransfersMara Jnelle100% (1)
- Microbiology Lab SafetyDocument5 pagesMicrobiology Lab Safetyrenz bartolomeNo ratings yet
- Exercise 3 - TRANSFER AND COLONY SELECTION TECHNIQUESDocument13 pagesExercise 3 - TRANSFER AND COLONY SELECTION TECHNIQUESArslan AmeenNo ratings yet
- Biohazard Material SpillsDocument3 pagesBiohazard Material SpillsrajeshbhramaNo ratings yet
- SOP For Staphylococcus AureusDocument4 pagesSOP For Staphylococcus AureusDr. StormNo ratings yet
- Culturing Bacteria from the EnvironmentDocument7 pagesCulturing Bacteria from the EnvironmentSer Louis Fetilo FabunanNo ratings yet
- Act. 3B Staining Methods For Microorganisms 2023Document8 pagesAct. 3B Staining Methods For Microorganisms 2023Mohamidin MamalapatNo ratings yet
- Laboratory Exercise 2. Aseptic TechniquesDocument7 pagesLaboratory Exercise 2. Aseptic TechniquesNesly Joy CaballeganNo ratings yet
- LAB WEEK 4 Gram StainingDocument4 pagesLAB WEEK 4 Gram Stainingtonistack847No ratings yet
- Exp. 10A - Aseptic TechniqueDocument5 pagesExp. 10A - Aseptic TechniqueMuchiri E MainaNo ratings yet
- Aseptic TechniquesDocument26 pagesAseptic TechniquesDonzzkie DonNo ratings yet
- XBMB3104 - SOALAN AMALI - MicrobiologyDocument19 pagesXBMB3104 - SOALAN AMALI - Microbiologykimi skyNo ratings yet
- Otprotocols 140307000050 Phpapp02Document76 pagesOtprotocols 140307000050 Phpapp02Melba AlanoNo ratings yet
- XBMB3104 (Microbiology) - Lab Sheet Sept 2022Document17 pagesXBMB3104 (Microbiology) - Lab Sheet Sept 2022Muhammad Sufri SalimunNo ratings yet
- Activity No. 1Document5 pagesActivity No. 1palamosapriltorresNo ratings yet
- Preparing Culture Media and Aseptic TechniqueDocument7 pagesPreparing Culture Media and Aseptic TechniqueNur AsiahNo ratings yet
- Surgical Aspesis (Lecture)Document4 pagesSurgical Aspesis (Lecture)ALLYSON GENE GULANESNo ratings yet
- ASPECTIC TECHNIQUES - NHF TrainingDocument7 pagesASPECTIC TECHNIQUES - NHF TrainingAlexia OwenNo ratings yet
- Carlos Vidaca - Where Oh Where Are You Hiding Bacteria Lab ReportDocument7 pagesCarlos Vidaca - Where Oh Where Are You Hiding Bacteria Lab Reportapi-391257001No ratings yet
- Aseptic Transfer TechniqueDocument13 pagesAseptic Transfer TechniqueYshaReyesNo ratings yet
- Safety Measurs in Microbiology Methods (Laboratory Rules)Document3 pagesSafety Measurs in Microbiology Methods (Laboratory Rules)Dr. Sujeet K. MritunjayNo ratings yet
- Aseptic Technique Lab ReviewDocument3 pagesAseptic Technique Lab ReviewAngelene GarciaNo ratings yet
- ASEPTIC PREPARATION COMPETENCY QUIZTITLE IV ROOM STERILE COMPOUNDING SKILLS ASSESSMENT TITLE ASSESSING ASEPTIC TECHNIQUE AND IV SAFETY KNOWLEDGEDocument5 pagesASEPTIC PREPARATION COMPETENCY QUIZTITLE IV ROOM STERILE COMPOUNDING SKILLS ASSESSMENT TITLE ASSESSING ASEPTIC TECHNIQUE AND IV SAFETY KNOWLEDGESherif KamalNo ratings yet
- Experiment 2 Aseptic TechniqueDocument30 pagesExperiment 2 Aseptic TechniqueAskYahGirl ChannelNo ratings yet
- Standard Operating Procedures (SOP) For B360C BSL2 Cell Culture RoomDocument4 pagesStandard Operating Procedures (SOP) For B360C BSL2 Cell Culture RoomvisiniNo ratings yet
- Grade 8 Module Week 3Document8 pagesGrade 8 Module Week 3Ivy MontecalvoNo ratings yet
- Project 1.2.3: Attack of The Superbugs: Streptococcus Pneumoniae, Commonly Referred To As Pneumococcus, Is A BacteriumDocument11 pagesProject 1.2.3: Attack of The Superbugs: Streptococcus Pneumoniae, Commonly Referred To As Pneumococcus, Is A BacteriumSeth WilkinsonNo ratings yet
- Activity 2:: Disinfection/Decontamiation of Working AreaDocument3 pagesActivity 2:: Disinfection/Decontamiation of Working AreaBianca GeagoniaNo ratings yet
- Easy Peasy Homemade Sanitizer Wipes: A Quick Guide with Visuals for Making Disposable Sanitizing Wipes Against MicrobesFrom EverandEasy Peasy Homemade Sanitizer Wipes: A Quick Guide with Visuals for Making Disposable Sanitizing Wipes Against MicrobesNo ratings yet
- Crosstabs: Case Processing SummaryDocument8 pagesCrosstabs: Case Processing SummarykhesiaNo ratings yet
- CHAPTER 4-Atty AliboghaDocument48 pagesCHAPTER 4-Atty AliboghaPaul EspinosaNo ratings yet
- Surfactantreplacementtherapy 160926164838Document33 pagesSurfactantreplacementtherapy 160926164838revathidadam55555No ratings yet
- GPPB Resolution No. 05-2020Document4 pagesGPPB Resolution No. 05-2020Villa BernaldoNo ratings yet
- PETTERUTI, WALSH & VELÁZQUEZ The Costs of Confinement Why Good Juvenile Justice Policies Make Good Fiscal SenseDocument25 pagesPETTERUTI, WALSH & VELÁZQUEZ The Costs of Confinement Why Good Juvenile Justice Policies Make Good Fiscal SenseFrancisco EstradaNo ratings yet
- SOP For Fumigation in Microbiology Lab - Pharmaceutical GuidelinesDocument1 pageSOP For Fumigation in Microbiology Lab - Pharmaceutical GuidelinesranaNo ratings yet
- Implementasi SPM Kesehatan Bayi Baru Lahir di Puskesmas DukuhsetiDocument10 pagesImplementasi SPM Kesehatan Bayi Baru Lahir di Puskesmas DukuhsetiFidia sariNo ratings yet
- Glasgow Coma Scale Canvas NCM 116 SkillsDocument6 pagesGlasgow Coma Scale Canvas NCM 116 Skillsbing bongNo ratings yet
- Comparative Quantification of Health RisksDocument2,258 pagesComparative Quantification of Health RisksHectorNo ratings yet
- Language Development: - Iphy OkoyeDocument9 pagesLanguage Development: - Iphy OkoyeIPHY OKOYENo ratings yet
- Ab Dental Catalogo 2017Document82 pagesAb Dental Catalogo 2017ilich sevillaNo ratings yet
- Handwashing Procedures GuideDocument8 pagesHandwashing Procedures GuideRizwanaNo ratings yet
- Ip 242 - 83-Schoniger Oxygen Flask Method PDFDocument3 pagesIp 242 - 83-Schoniger Oxygen Flask Method PDFPedro AluaNo ratings yet
- Master Nauli for a Strong Core and DigestionDocument7 pagesMaster Nauli for a Strong Core and DigestionOVVCMOULINo ratings yet
- Risk and Associated Factors of Depression and AnxiDocument27 pagesRisk and Associated Factors of Depression and AnxiDian Oktaria SafitriNo ratings yet
- AP LacrimalDocument38 pagesAP LacrimalIoana MovileanuNo ratings yet
- Dr. Nirali Patel Pediatric Emergency MedicineDocument31 pagesDr. Nirali Patel Pediatric Emergency Medicinemarsan12No ratings yet
- Original Article: Genetic Inheritance Pattern in Prurigo HebraDocument6 pagesOriginal Article: Genetic Inheritance Pattern in Prurigo HebraTaufiq AkbarNo ratings yet
- 05-OSH Promotion Training & CommunicationDocument38 pages05-OSH Promotion Training & Communicationbuggs115250% (2)
- SELF-EFFICACY THEORY SOURCESDocument14 pagesSELF-EFFICACY THEORY SOURCESagungNo ratings yet
- Notes On Psychology of DisabilityDocument12 pagesNotes On Psychology of DisabilityLavKanya 98No ratings yet
- DLL - Mapeh 4 - Q2 - W8Document6 pagesDLL - Mapeh 4 - Q2 - W8Amy Santillan PanisalNo ratings yet
- Full Download Ebook Ebook PDF Nutrition Therapy and Pathophysiology 4th Edition PDFDocument51 pagesFull Download Ebook Ebook PDF Nutrition Therapy and Pathophysiology 4th Edition PDFbessie.skubis114100% (42)