Professional Documents
Culture Documents
Vein To Vein Diagram
Vein To Vein Diagram
Uploaded by
Prashant Tripathi0 ratings0% found this document useful (0 votes)
6 views2 pagesThis document describes the process for manufacturing and delivering NexCAR19, a CAR T-cell therapy. The process involves:
1. Extracting a patient's white blood cells through leukapheresis at the hospital.
2. Shipping the extracted cells to the manufacturing site for selection, activation, and genetic modification of T-cells to target B-cell cancer.
3. Testing and cryopreserving the manufactured product before shipping back to the hospital within 9-19 days for intravenous infusion into the patient.
Original Description:
Car t cells therapy for cancer treatment
Original Title
Vein to Vein diagram
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document describes the process for manufacturing and delivering NexCAR19, a CAR T-cell therapy. The process involves:
1. Extracting a patient's white blood cells through leukapheresis at the hospital.
2. Shipping the extracted cells to the manufacturing site for selection, activation, and genetic modification of T-cells to target B-cell cancer.
3. Testing and cryopreserving the manufactured product before shipping back to the hospital within 9-19 days for intravenous infusion into the patient.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views2 pagesVein To Vein Diagram
Vein To Vein Diagram
Uploaded by
Prashant TripathiThis document describes the process for manufacturing and delivering NexCAR19, a CAR T-cell therapy. The process involves:
1. Extracting a patient's white blood cells through leukapheresis at the hospital.
2. Shipping the extracted cells to the manufacturing site for selection, activation, and genetic modification of T-cells to target B-cell cancer.
3. Testing and cryopreserving the manufactured product before shipping back to the hospital within 9-19 days for intravenous infusion into the patient.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
TM
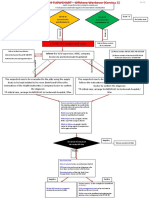
NexCAR19
Actalycabtagene autoleucel
From Vein-to-Vein
Hospital Manufacturing Site
Patient consent &
enrollment
Day 0 Shipping Day 1 - Day 9
Leukapheresis (The Leukopak is shipped Manufacturing
(White Blood Cells to ImmunoACT’s (ImmunoACT’s scientists select and activate the T-cells, then modify them using a safe
are extracted by a manufacturing facility lentiviral vector. This makes the T-cells express special connectors, called Chimeric Antigen
machine at the under refrigerated Receptors (CARs) that target your B-cell cancer. The cells are then cultured and expanded
Hospital, in a conditions) until they reach a target dose)
Leukopak)
Product Quality Formulation and
Assurance Cryopreservation
Infusion Day 9-19
(The Product is administered via Shipping
intravenous infusion. Prior to this, (The Product is shipped in (The Product is cryopreserved, and
the patient is given a conditioning an Infusion bag under undergoes extensive testing to ensure its
chemotherapy to ready their body cryopreserved conditions to Identity, Purity, Safety, & Potency)
for the treatment) the hospital)
You might also like
- Checklist For Best Practices For Vaccination Clinics Held at Satellite, Temporary, or Off-Site LocationsDocument6 pagesChecklist For Best Practices For Vaccination Clinics Held at Satellite, Temporary, or Off-Site LocationsMark Philipp AbanNo ratings yet
- 6 Benefits of GarlicDocument1 page6 Benefits of GarlicDetteNo ratings yet
- QC Stock Organism Maintenance ProgramDocument3 pagesQC Stock Organism Maintenance ProgramLiana PergisNo ratings yet
- Direct Method: Urogenital Mycoplasma DiagnosisDocument2 pagesDirect Method: Urogenital Mycoplasma DiagnosisAnel RedzicNo ratings yet
- I Vancomycin: SystemDocument7 pagesI Vancomycin: Systemtesteste testeNo ratings yet
- Vancomycin ARCDocument7 pagesVancomycin ARCtesteteee testtesteteNo ratings yet
- Thymovac - Product IntroductionDocument20 pagesThymovac - Product Introductionnquockhanh1998No ratings yet
- Covid-19 Vaccine (Vero Cell), Inactivated: Coronavac March, 2021Document38 pagesCovid-19 Vaccine (Vero Cell), Inactivated: Coronavac March, 2021Abu HumairaNo ratings yet
- Produksi VaksinDocument25 pagesProduksi Vaksinkehie HananielNo ratings yet
- Ivermectin Potential Candidate For The Treatment of Covid 19Document3 pagesIvermectin Potential Candidate For The Treatment of Covid 19glenwell sentosaNo ratings yet
- Korean Biopharmas Are Striving To Accelerate Expansion Into The Global Market by FormingDocument1 pageKorean Biopharmas Are Striving To Accelerate Expansion Into The Global Market by FormingRicky ChiuNo ratings yet
- VVT Process Flow Overview BrochureDocument2 pagesVVT Process Flow Overview BrochureThu PhamNo ratings yet
- Oncology Pharmacy DesignDocument1 pageOncology Pharmacy Designdinand_sNo ratings yet
- Brosur Clinitek Novus BrochureDocument2 pagesBrosur Clinitek Novus BrochureFarly AugusNo ratings yet
- 2002 Produccion de Ab Monoclonales en Plntas TransgenicasDocument6 pages2002 Produccion de Ab Monoclonales en Plntas TransgenicasSilvia MamaniNo ratings yet
- PosterDocument1 pagePosterP BNo ratings yet
- Infliximab PosterDocument1 pageInfliximab Posterapi-355090691No ratings yet
- 10.1021@cen 09409 Buscon008Document1 page10.1021@cen 09409 Buscon008Amal ..No ratings yet
- 24-4-2018-Mats LundgrenDocument22 pages24-4-2018-Mats Lundgrensushaantb400No ratings yet
- 1 (6) - 部分18Document1 page1 (6) - 部分18Ricky ChiuNo ratings yet
- 1 (6) - 部分26Document1 page1 (6) - 部分26Ricky ChiuNo ratings yet
- Surgical Cure of Clarithromycin Resistant Mycoba - 2020 - Journal of Clinical TuDocument5 pagesSurgical Cure of Clarithromycin Resistant Mycoba - 2020 - Journal of Clinical TuKarina ChristantoNo ratings yet
- Veno and LidoDocument6 pagesVeno and LidoIRA MONIQUE CABADENNo ratings yet
- Procedimento Implante ActiconDocument28 pagesProcedimento Implante Acticonapi-3762376No ratings yet
- 62 Microbiological Pseudomonas Aeruginosa Such As Atcc 9027 NcimbDocument5 pages62 Microbiological Pseudomonas Aeruginosa Such As Atcc 9027 NcimbMario MartínezNo ratings yet
- Amgen Pharmaceutical Manufacturing Facility, West Greenwich Rhode Island - Pharmaceutical TechnologyDocument4 pagesAmgen Pharmaceutical Manufacturing Facility, West Greenwich Rhode Island - Pharmaceutical Technologysarfaraz_niaziNo ratings yet
- Best Practices For Sample Collection, Packaging, Transportation, and TestingDocument28 pagesBest Practices For Sample Collection, Packaging, Transportation, and TestingvgmanjunathNo ratings yet
- Proteins For Neuroscience AneuroDocument17 pagesProteins For Neuroscience AneuroDevrim CK ÇetinkayalıNo ratings yet
- Sop - 22 - V3Document4 pagesSop - 22 - V3John Francis RosasNo ratings yet
- IVF Store BrandsDocument5 pagesIVF Store BrandsNasraldeen MohamedNo ratings yet
- Bioreactor Concepts For Cell Culture-Based Viral Vaccine ProductionDocument15 pagesBioreactor Concepts For Cell Culture-Based Viral Vaccine ProductionJohn Lloyd GenerosoNo ratings yet
- Antibiotics Currently in Development April 2020Document36 pagesAntibiotics Currently in Development April 2020tanishtarun06No ratings yet
- 10.1016@j.jconrel.2018.06.014Document10 pages10.1016@j.jconrel.2018.06.014Shalin CNo ratings yet
- Medical Device Market Research Report: Fall 2008Document40 pagesMedical Device Market Research Report: Fall 2008DougNo ratings yet
- Clinical Potential of A Silk Sericin-Releasing Bioactive Wound Dressing For The Treatment of Split-Thickness Skin Graft Donor SitesDocument14 pagesClinical Potential of A Silk Sericin-Releasing Bioactive Wound Dressing For The Treatment of Split-Thickness Skin Graft Donor SitesAgus DniNo ratings yet
- Presentation Manufacturing Process Biologics Kowid Ho Afssaps enDocument30 pagesPresentation Manufacturing Process Biologics Kowid Ho Afssaps enJ Diaz100% (1)
- Very Large Scale Monoclonal Antibody Purification Kelley 2007Document14 pagesVery Large Scale Monoclonal Antibody Purification Kelley 2007dhaya777No ratings yet
- IF Guide 2021Document36 pagesIF Guide 2021Mario RodriguezNo ratings yet
- Angeles University Foundation: I) Clinical PathologyDocument20 pagesAngeles University Foundation: I) Clinical PathologyKaye ManalastasNo ratings yet
- BMJ O926 FullDocument3 pagesBMJ O926 FullMonika Diaz KristyanindaNo ratings yet
- 62 Microbiological Examination of Nonsterile Products: Tests For Specified MicroorganismsDocument5 pages62 Microbiological Examination of Nonsterile Products: Tests For Specified Microorganismsmustea_ana9616No ratings yet
- Adenoviral Vector COVID-19 VaccinesDocument22 pagesAdenoviral Vector COVID-19 VaccinesVALENTINA CARRILLO BENAVIDEZNo ratings yet
- Corneal Delivery of Besifloxacin Polymeric MicroniddlesDocument11 pagesCorneal Delivery of Besifloxacin Polymeric MicroniddlesDaisy Arora KhuranaNo ratings yet
- Overview of Biotechnology at The End of The 20th CenturyDocument11 pagesOverview of Biotechnology at The End of The 20th Centuryemaemars92No ratings yet
- COVID-19 Suspected Case: Inform The W/O Supervisor, HSSE, CompanyDocument1 pageCOVID-19 Suspected Case: Inform The W/O Supervisor, HSSE, CompanybilouNo ratings yet
- Pharmaceuticals & Medical Supplies: Amarila Malik Professor, Faculty of Pharmacy - Universitas IndonesiaDocument22 pagesPharmaceuticals & Medical Supplies: Amarila Malik Professor, Faculty of Pharmacy - Universitas IndonesiaAngga AnugrawanNo ratings yet
- NOVOMIXDocument3 pagesNOVOMIXAsri Putri PratiwiNo ratings yet
- GenericsDocument1 pageGenericsvikasbansal227No ratings yet
- Introducing SPW's "We Are Thinking" SeriesDocument3 pagesIntroducing SPW's "We Are Thinking" Seriesliz knightNo ratings yet
- Beckman ReagentsDocument8 pagesBeckman ReagentskadirucaNo ratings yet
- Logix Smart COVID 19 IVD Brochure CE and EUA Rev - 1Document2 pagesLogix Smart COVID 19 IVD Brochure CE and EUA Rev - 1Luis UribeNo ratings yet
- Yisheng Biopharma Profile For CandidatesDocument9 pagesYisheng Biopharma Profile For CandidatesKeith SummerNo ratings yet
- CTX Solutions Fact Sheet WebDocument2 pagesCTX Solutions Fact Sheet WebAkhan MukhanovNo ratings yet
- CTX Solutions Fact Sheet WebDocument2 pagesCTX Solutions Fact Sheet WebAkhan MukhanovNo ratings yet
- Company Profile Kalgen Innolab Innobiogram - 2022Document12 pagesCompany Profile Kalgen Innolab Innobiogram - 2022asmadi arrumNo ratings yet
- Data Briefing Slides 11112020Document3 pagesData Briefing Slides 11112020exeri0nNo ratings yet
- Technical Tip: Irradiation Glossary of TermsDocument2 pagesTechnical Tip: Irradiation Glossary of TermsRakeshNo ratings yet
- Manufacturing of Pfizer Covid VaccineDocument3 pagesManufacturing of Pfizer Covid VaccineCharlie MaineNo ratings yet
- Temperature Controlled Cargo Operation NewDocument121 pagesTemperature Controlled Cargo Operation NewawdireNo ratings yet
- Ios Assignmnt Prashant Tripathi Ity BranchDocument8 pagesIos Assignmnt Prashant Tripathi Ity BranchPrashant TripathiNo ratings yet
- Human Right Council PPT Prashant Tripathi Roll No 34 CBDocument9 pagesHuman Right Council PPT Prashant Tripathi Roll No 34 CBPrashant TripathiNo ratings yet
- Attendance Registre: .. Name of The MonthDocument5 pagesAttendance Registre: .. Name of The MonthPrashant TripathiNo ratings yet
- Acknowledgement & Certificate.Document2 pagesAcknowledgement & Certificate.Prashant TripathiNo ratings yet