Professional Documents
Culture Documents
Selectivity and Reactivity in Enolate Reactions - Answer Key
Selectivity and Reactivity in Enolate Reactions - Answer Key
Uploaded by
yavaxoh9350 ratings0% found this document useful (0 votes)
3 views3 pagesThe document discusses selectivity and reactivity in enolate reactions and poses three questions. The first question asks for the products of reactions involving a diketone substrate with KOH, H3O+, and no base. The second question asks to show the step-by-step mechanism for a transformation. The third question asks for the step-by-step mechanism of a 1,4-addition reaction using OH- as the base. General advice is given to familiarize yourself with nucleophiles that do 1,2 and 1,4 additions to alpha,beta-unsaturated carbonyls.
Original Description:
Original Title
Selectivity and Reactivity in Enolate Reactions_Answer Key
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses selectivity and reactivity in enolate reactions and poses three questions. The first question asks for the products of reactions involving a diketone substrate with KOH, H3O+, and no base. The second question asks to show the step-by-step mechanism for a transformation. The third question asks for the step-by-step mechanism of a 1,4-addition reaction using OH- as the base. General advice is given to familiarize yourself with nucleophiles that do 1,2 and 1,4 additions to alpha,beta-unsaturated carbonyls.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
3 views3 pagesSelectivity and Reactivity in Enolate Reactions - Answer Key
Selectivity and Reactivity in Enolate Reactions - Answer Key
Uploaded by
yavaxoh935The document discusses selectivity and reactivity in enolate reactions and poses three questions. The first question asks for the products of reactions involving a diketone substrate with KOH, H3O+, and no base. The second question asks to show the step-by-step mechanism for a transformation. The third question asks for the step-by-step mechanism of a 1,4-addition reaction using OH- as the base. General advice is given to familiarize yourself with nucleophiles that do 1,2 and 1,4 additions to alpha,beta-unsaturated carbonyls.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 3
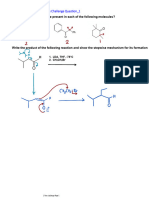
Selectivity and Reactivity in Enolate Reactions Challenge
Question_1
Give the product of each of the following reactions:
1. KOH
2.
3. H3O+
25-day challenge Page 1
Selectivity and Reactivity in Enolate Reactions Challenge
Question_2
Show how you would make the following transformation and write the step-by-step mechanism.
25-day challenge Page 2
Selectivity and Reactivity in Enolate Reactions Challenge
Question_3
Write the step-by-step mechanism for the following reaction
The answer is the same as in Q2, except that here, OH- is the base. Form the
stabilized enolate from the 1,3-dicarbonyl, then make it do a 1,4-addition to the
α,β-unsaturated carbonyl. It is a Robinson Annulation again.
As general reminder when it comes to α,β-
unsaturated carbonyls, familiarize yourself with
reagents (Nucleophiles) that do the 1,2 (direct
addition) and those that do the 1,4 (conjugate)
addition
25-day challenge Page 3
You might also like
- SLG Chem3 LG 2.12 Elimination Reactions (E1 and E2Document6 pagesSLG Chem3 LG 2.12 Elimination Reactions (E1 and E2Lorraine CalacsanNo ratings yet
- Chemistry-Nucleophilic and Free Radical AdditionDocument12 pagesChemistry-Nucleophilic and Free Radical Additionrakeshtrikha8668No ratings yet
- CHEM 1701 - Lab 6 - Types of Reactions & Redox: Chemistry I For Pre-Health SciencesDocument5 pagesCHEM 1701 - Lab 6 - Types of Reactions & Redox: Chemistry I For Pre-Health Sciencesapi-535522887No ratings yet
- TB Chapter11Document11 pagesTB Chapter11Luke SkywalkerNo ratings yet
- (L2) - (JLD 4.0) - Alkyl Halide - 18th OctDocument77 pages(L2) - (JLD 4.0) - Alkyl Halide - 18th Octdinesh.mission.iitNo ratings yet
- Assignment 4 BLC F20Document2 pagesAssignment 4 BLC F20Rémi MartineauNo ratings yet
- Orm IiiDocument53 pagesOrm Iiilopa39018No ratings yet
- 2562726-Class 12 - Unit Test - Chemistry - Set 1 - Jenifer - QPDocument4 pages2562726-Class 12 - Unit Test - Chemistry - Set 1 - Jenifer - QPkjfnk,jgNo ratings yet
- CHEM1002 - Workshop 12Document14 pagesCHEM1002 - Workshop 12KHÁNH VÂN DIỆPNo ratings yet
- Organic Reaction Mechanisms-Iii Ontents: JEE (Advanced) SyllabusDocument59 pagesOrganic Reaction Mechanisms-Iii Ontents: JEE (Advanced) SyllabusGOURISH AGRAWAL75% (4)
- phản ứng aldehydeDocument5 pagesphản ứng aldehydeTrungTâmYaSaNo ratings yet
- Carbonyl-Based Nucleophiles Challenge Question - 1Document3 pagesCarbonyl-Based Nucleophiles Challenge Question - 1yavaxoh935No ratings yet
- Alkyl Halide QuestionsDocument6 pagesAlkyl Halide QuestionsAaron Lee100% (1)
- Notes in Limiting Reactant Day4Document3 pagesNotes in Limiting Reactant Day4Olga AsiaNo ratings yet
- (Template) Hydrocarbon TestDocument3 pages(Template) Hydrocarbon Test텅텅No ratings yet
- Chemistry Test - 12th Science-ChemistryDocument7 pagesChemistry Test - 12th Science-ChemistryAishley ChalametNo ratings yet
- 4Q Sci10 Las5 Chemical ReactionsDocument4 pages4Q Sci10 Las5 Chemical Reactionsrectoann08No ratings yet
- 17b. Alkenes Homework (2 of 3)Document6 pages17b. Alkenes Homework (2 of 3)Haz PlayzNo ratings yet
- Quiz Submissions - Mastery Quiz 5 - Chemical Reactions Weeks 10 11 Content - 202241Document7 pagesQuiz Submissions - Mastery Quiz 5 - Chemical Reactions Weeks 10 11 Content - 202241api-607515535No ratings yet
- CHEM 2425 Review For Test 4 - Chapter 22 - 23 - 24 - 230115 - 183827Document21 pagesCHEM 2425 Review For Test 4 - Chapter 22 - 23 - 24 - 230115 - 183827mahdi aliNo ratings yet
- Questions For PracticeDocument6 pagesQuestions For PracticeAshmitNo ratings yet
- Aakash Institute: NCERT Solutions For Class 12 Chemistry Chapter 11 Alcohols, Phenols and EthersDocument52 pagesAakash Institute: NCERT Solutions For Class 12 Chemistry Chapter 11 Alcohols, Phenols and EthersHARSHALNo ratings yet
- Nucleophilic Substitution Reactions PDFDocument13 pagesNucleophilic Substitution Reactions PDFBhushan Dravyakar100% (6)
- Using Molecular Models To Represent Chemical ReactionsDocument3 pagesUsing Molecular Models To Represent Chemical ReactionsAndrea Tamez ContrerasNo ratings yet
- Ch11 LeeDocument19 pagesCh11 LeeRameRed LiNo ratings yet
- (JPP-4) - (JLD 3.0) - Reaction Mechanisms - 30 AugDocument50 pages(JPP-4) - (JLD 3.0) - Reaction Mechanisms - 30 Augydouneed2012No ratings yet
- 03 Chapter 3 PostDocument77 pages03 Chapter 3 PostPhan ThongNo ratings yet
- POGIL Net Ionic Equations - SDocument5 pagesPOGIL Net Ionic Equations - Sdemyeets64No ratings yet
- Module 6 General Types of Organic ReactionsDocument12 pagesModule 6 General Types of Organic ReactionsCielo DasalNo ratings yet
- Correct Not Sure No AnswerDocument31 pagesCorrect Not Sure No Answerblahblah nyenyeNo ratings yet
- CHEM 1701 - Lab 6 - Types of Reactions & Redox - /10 MarksDocument5 pagesCHEM 1701 - Lab 6 - Types of Reactions & Redox - /10 Marksapi-535593780No ratings yet
- Echem 001 - g4 Lab Rep 3.2Document7 pagesEchem 001 - g4 Lab Rep 3.2RHANE CATRINE CORREANo ratings yet
- CHAPTER 7 - Haloalkanes PDFDocument42 pagesCHAPTER 7 - Haloalkanes PDFPaolo NaguitNo ratings yet
- Science General Chemistry 1: Whole Brain Learning System Outcome-Based EducationDocument20 pagesScience General Chemistry 1: Whole Brain Learning System Outcome-Based EducationKayrell AquinoNo ratings yet
- Organic Reaction Mechanisms-Iv Ontents: JEE (Advanced) SyllabusDocument43 pagesOrganic Reaction Mechanisms-Iv Ontents: JEE (Advanced) SyllabusGOURISH AGRAWAL100% (2)
- Ncert Solutions March9 For Class 11 Chemistry Chapter 8Document52 pagesNcert Solutions March9 For Class 11 Chemistry Chapter 8Pushpit jainNo ratings yet
- Alkenes ReactionsDocument22 pagesAlkenes Reactions04Andhika FathurrohmanNo ratings yet
- 4818 Et EtDocument10 pages4818 Et EtAshuNo ratings yet
- Chem12 C1102 SWBSDocument5 pagesChem12 C1102 SWBSAhmad asaNo ratings yet
- Chem102 Chemical Kinetics 3Document22 pagesChem102 Chemical Kinetics 3Zabo TrewNo ratings yet
- Organic Chemistry With Biological Applications 3Rd Edition Mcmurry Test Bank Full Chapter PDFDocument36 pagesOrganic Chemistry With Biological Applications 3Rd Edition Mcmurry Test Bank Full Chapter PDFmisstepmonocarp1b69100% (10)
- Alkylhalide ReactionsDocument36 pagesAlkylhalide ReactionsTobenna100% (1)
- April 9thDocument3 pagesApril 9thapi-697899968No ratings yet
- 11 - NotesDocument12 pages11 - Notescynthiamei888No ratings yet
- Problem 2-Kolbe Reaction: Created by Hanna Key Grinnell CollegeDocument1 pageProblem 2-Kolbe Reaction: Created by Hanna Key Grinnell CollegeShyam BhaktaNo ratings yet
- Kinetics and Mechanisms POGILDocument6 pagesKinetics and Mechanisms POGILElainaNo ratings yet
- CH 07Document42 pagesCH 07Samantha Louise MondonedoNo ratings yet
- Section B Answer All Questions in The Spaces Provided: Table 1Document5 pagesSection B Answer All Questions in The Spaces Provided: Table 1Sweetie AndersonNo ratings yet
- CHEM 1701 - Lab 6 - Chemical Reactions: Chemistry I For Pre-Health Sciences (Online)Document7 pagesCHEM 1701 - Lab 6 - Chemical Reactions: Chemistry I For Pre-Health Sciences (Online)api-534361913No ratings yet
- CHEM 2321 Section 7 Halides W23Document34 pagesCHEM 2321 Section 7 Halides W23thathukieuthuyNo ratings yet
- Alkenes and AlkynesDocument2 pagesAlkenes and AlkynesLewis AlfonsoNo ratings yet
- The 5 Types of Chemical ReactionsDocument20 pagesThe 5 Types of Chemical ReactionsElfara PuspitaNo ratings yet
- Chapter 7Document37 pagesChapter 7민규강No ratings yet
- 26.12.19sprint-Alcohol Phenol EtherDocument55 pages26.12.19sprint-Alcohol Phenol Ethersnehasngh909No ratings yet
- 5.1.1 How Fast QPDocument25 pages5.1.1 How Fast QPSir MannyNo ratings yet
- KEC - Lecture-VIII-PST - BCE B-078-03-23 - 1662274112Document20 pagesKEC - Lecture-VIII-PST - BCE B-078-03-23 - 1662274112bsarad115No ratings yet
- Experiment 5 Reaction of Hydroxy Compounds: SK025 Pre-Lab ModuleDocument3 pagesExperiment 5 Reaction of Hydroxy Compounds: SK025 Pre-Lab Modulenur anis amirahNo ratings yet
- CHEM 1701 - Lab 6 - Chemical Reactions: Chemistry I For Pre-Health Sciences (Online)Document7 pagesCHEM 1701 - Lab 6 - Chemical Reactions: Chemistry I For Pre-Health Sciences (Online)api-547526297No ratings yet
- Mechanisms1Document14 pagesMechanisms1Anthony Mafuta MayilameneNo ratings yet
- Mike 1212Document1 pageMike 1212yavaxoh935No ratings yet
- TESTTESTestwsdfsawi Dfaodfj Asd As Das Dasd SDF SdfasDocument2 pagesTESTTESTestwsdfsawi Dfaodfj Asd As Das Dasd SDF Sdfasyavaxoh935No ratings yet
- Interview 1qDocument1 pageInterview 1qyavaxoh935No ratings yet
- CycloaddDocument3 pagesCycloaddyavaxoh935No ratings yet
- TESTTESTestwsdfsawi Dfaodfj Asd As Das Daasd ZXDDocument2 pagesTESTTESTestwsdfsawi Dfaodfj Asd As Das Daasd ZXDyavaxoh935No ratings yet
- Carbonyl-Based Nucleophiles Challenge Question - 1Document3 pagesCarbonyl-Based Nucleophiles Challenge Question - 1yavaxoh935No ratings yet
- ElimisDocument3 pagesElimisyavaxoh935No ratings yet