Professional Documents
Culture Documents
Doxycycline Prolonged Release Capsules
Uploaded by
Alexandra CociuOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Doxycycline Prolonged Release Capsules
Uploaded by
Alexandra CociuCopyright:
Available Formats
Doxycycline Prolonged-release Capsules – BP 2019
These chromatograms are provided for information only as an aid to analysts and are intended as
guidance for the interpretation and application of BP monographs.
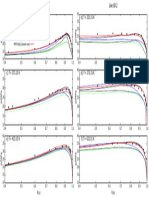
Typical chromatogram for solution (3) in the Related Substances test for Doxycycline Prolonged-release
Capsules as published in BP 2019.
12 12
10 10
8 8
6 6
mAU
mAU
4 1 4
2 2
0 0
4
0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44
Minutes
Peak ID: 1: Impurity C; 2: Impurity A; 3: Impurity B; 4: Doxycycline; 5: Impurity F
Column : Waters, XTerra RP18 (250 mm x 4.6 mm, 5 μm)
Solution A : 11.16 % w/v solution of ethylenediaminetetracetic acid disodium salt dihydrate,
adjusted to pH 7.0 with concentrated ammonia (35 %)
Buffer : 6.79 % w/v tetrabutylammonium hydrogen sulfate solution adjusted to pH 7.0
with concentrated ammonia (35 %)
Mobile Phase : Acetonitrile: water: buffer: solution A (13: 17: 35: 35, v/v/v/v)
Diluent : 0.01 M hydrochloric acid
Flow Rate : 1 mL/min
Column Temp : 35°C
Injection Volume : 20 µL
Detection : UV, 280 nm
Typical chromatogram for solution (2) in the Assay test for Doxycycline Prolonged-release Capsules as
published in BP 2019.
35 35
30 30
25 25
20 20
mAU
mAU
15 15
10 10
5 5
0 0
1
0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30
Minutes
Peak ID: 1: Doxycycline
Column : Waters, XTerra RP18 (250 mm x 4.6 mm, 5 μm)
Solution A : 11.16 % w/v solution of ethylenediaminetetracetic acid disodium salt dihydrate,
adjusted to pH 7.0 with concentrated ammonia (35 %)
Buffer : 6.79 % w/v tetrabutylammonium hydrogen sulfate solution adjusted to pH 7.0
with concentrated ammonia (35 %)
Mobile Phase : Acetonitrile: water: buffer: solution A (13: 17: 35: 35, v/v/v/v)
Diluent : 0.01 M hydrochloric acid.
Flow Rate : 1 mL/min
Column Temp : 35°C
Injection Volume : 20 µL
Detection : UV, 280 nm
You might also like
- The Fusion Marketing Bible: Fuse Traditional Media, Social Media, & Digital Media to Maximize MarketingFrom EverandThe Fusion Marketing Bible: Fuse Traditional Media, Social Media, & Digital Media to Maximize MarketingRating: 5 out of 5 stars5/5 (2)
- Doxycycline TabletsDocument2 pagesDoxycycline TabletsAlexandra CociuNo ratings yet
- The Toyota Kata Practice Guide: Practicing Scientific Thinking Skills for Superior Results in 20 Minutes a DayFrom EverandThe Toyota Kata Practice Guide: Practicing Scientific Thinking Skills for Superior Results in 20 Minutes a DayRating: 4.5 out of 5 stars4.5/5 (7)
- Laborator 1Document1 pageLaborator 1Last HeroNo ratings yet
- CSTR y PFRDocument3 pagesCSTR y PFREugenio Sebastian AndradeNo ratings yet
- PreviewDocument2 pagesPreviewCon Bò Sữa Thất TìnhNo ratings yet
- Pump CurveDocument8 pagesPump CurveClark HonradoNo ratings yet
- Classeur 2Document2 pagesClasseur 2Twin KileNo ratings yet
- Teams Oppurtunities Target Actual Defects %Document4 pagesTeams Oppurtunities Target Actual Defects %ramaiahgantaNo ratings yet
- Exp 3 - Centrifugal Pump ExperimentDocument7 pagesExp 3 - Centrifugal Pump ExperimentAnusha SinghNo ratings yet
- ATU V 1.0 February 2020 FinalDocument41 pagesATU V 1.0 February 2020 FinalMarcelo UGNo ratings yet
- 10 1007@s41742-018Document9 pages10 1007@s41742-018Radouane El-AmriNo ratings yet
- MC500EDocument23 pagesMC500EvicnitNo ratings yet
- Monthly Cases of Dengue Fever Admitted in Tubigon Community Hospital: Tubigon ResidentsDocument2 pagesMonthly Cases of Dengue Fever Admitted in Tubigon Community Hospital: Tubigon ResidentsGina BoligaoNo ratings yet
- PAK Transformer Study 15Nov2019NREL v2Document33 pagesPAK Transformer Study 15Nov2019NREL v2Asim RiazNo ratings yet
- Computer AssignmentDocument679 pagesComputer Assignmentjahanvi vermaNo ratings yet
- Curva Flygt HS 5100 MT 3 431-Tuberia 4 PulgDocument5 pagesCurva Flygt HS 5100 MT 3 431-Tuberia 4 PulgAlbert ChacoNo ratings yet
- Iset 0.25 Iset 0.5: MPS MPSDocument3 pagesIset 0.25 Iset 0.5: MPS MPSIQbàl Nak BhrocokNo ratings yet
- Condensate Accumulation CalculationDocument4 pagesCondensate Accumulation CalculationKevin PratyatamaNo ratings yet
- SCHWING TrainingManual (229 261)Document33 pagesSCHWING TrainingManual (229 261)Petr Kos67% (3)
- Iset 0.25 Iset 0.5: MPS MPSDocument3 pagesIset 0.25 Iset 0.5: MPS MPSIsmailNo ratings yet
- Ritonavir TabletsDocument3 pagesRitonavir TabletsTiana JovanovicNo ratings yet
- MP GPW1000 GB D F - 03 97Document84 pagesMP GPW1000 GB D F - 03 97RomanNo ratings yet
- HT 75 eDocument1 pageHT 75 eGiles HefferanNo ratings yet
- Crystal Lake - Curse GTR RDocument12 pagesCrystal Lake - Curse GTR RviviviviNo ratings yet
- Pnas 1922319117 SappDocument11 pagesPnas 1922319117 SappAbeyNo ratings yet
- Kontrol Kuantitatif: Diagram Kontrol Jangkauan (R-Chart)Document6 pagesKontrol Kuantitatif: Diagram Kontrol Jangkauan (R-Chart)DebbyLaksanaAnugrahNo ratings yet
- La Guia MetAs 10 06 Densidad AguaDocument19 pagesLa Guia MetAs 10 06 Densidad AguaMayreneDavilaNo ratings yet
- Uji Normalitas Shapiro WilkDocument10 pagesUji Normalitas Shapiro Wilktisya safaathinNo ratings yet
- Using The Shimadzu GC System in The Fuel-Grade Ethanol Production Laboratory. Application Note (Shimadzu)Document2 pagesUsing The Shimadzu GC System in The Fuel-Grade Ethanol Production Laboratory. Application Note (Shimadzu)Maikel Perez NavarroNo ratings yet
- DIN 2391-2 Cijevi SpecijalneDocument10 pagesDIN 2391-2 Cijevi SpecijalneMiran VidovićNo ratings yet
- Bio Oil Tebu 24 Jan 2022Document13 pagesBio Oil Tebu 24 Jan 2022Haniif PrasetyawanNo ratings yet
- Op Tim Ization 5Document1 pageOp Tim Ization 5Reza ArefidamghaniNo ratings yet
- Potenciometria y CromatografíaDocument7 pagesPotenciometria y Cromatografíanaomyabigail48No ratings yet
- Horus h59 Reticle Technical SpecsDocument5 pagesHorus h59 Reticle Technical SpecsThomas MpourtzalasNo ratings yet
- Structure Activity Relationship of Carbonic Anhydrase InhibitorsDocument10 pagesStructure Activity Relationship of Carbonic Anhydrase InhibitorsnatdempkowskiNo ratings yet
- Data Sheet KTZ47.5-53: Specification Shaft SealDocument4 pagesData Sheet KTZ47.5-53: Specification Shaft Sealheru heriyantoNo ratings yet
- Capitulo #8 8.2.-Flexion Biaxial en Columnas RectangularesDocument67 pagesCapitulo #8 8.2.-Flexion Biaxial en Columnas RectangularesJorge A Lau QNo ratings yet
- 2 UnidadDocument5 pages2 UnidadLuis Manuel Miguel AcostaNo ratings yet
- Data Sheet KTZ33.7-52: Specification Shaft SealDocument4 pagesData Sheet KTZ33.7-52: Specification Shaft SealMario VazquezNo ratings yet
- Grafik Sondir: Conus (KG/CM )Document5 pagesGrafik Sondir: Conus (KG/CM )ROSA PHETY PERMATASARINo ratings yet
- HidrografDocument5 pagesHidrografDexter MagisterNo ratings yet
- 02-10-6816 Hydr. Connections, Mast - h3 347 2Document1 page02-10-6816 Hydr. Connections, Mast - h3 347 2Juan Ismael Grave LolNo ratings yet
- Problemario Capítulo 6 Ingeniería de Procesos MicrobiológicosDocument25 pagesProblemario Capítulo 6 Ingeniería de Procesos MicrobiológicosKenia CarrilloNo ratings yet
- PC750-7 S/N 20001-UP (Overseas Version)Document2 pagesPC750-7 S/N 20001-UP (Overseas Version)АлександрNo ratings yet
- Gearbox PDFDocument2 pagesGearbox PDFeduardo chavezNo ratings yet
- Ridgid No. 460-6 TristandDocument1 pageRidgid No. 460-6 TristandenriqueNo ratings yet
- Figure 3.3: B Versus DWT Figure 3.4: D Versus DWT: DWT (T) DWT (T)Document2 pagesFigure 3.3: B Versus DWT Figure 3.4: D Versus DWT: DWT (T) DWT (T)ABHIROOP KNo ratings yet
- 4 (Parentship Graph)Document2 pages4 (Parentship Graph)ABHIROOP KNo ratings yet
- Useful Stuff ChartsDocument5 pagesUseful Stuff ChartsDevilish LuciferNo ratings yet
- Latihan Soal PeengukuranDocument3 pagesLatihan Soal PeengukuranMEI EDI PRAYITNO, STNo ratings yet
- Haemophilus Influenzae: Zone Diameter Breakpoints and Disk QC Criteria For and Ceftolozane-TazobactamDocument2 pagesHaemophilus Influenzae: Zone Diameter Breakpoints and Disk QC Criteria For and Ceftolozane-TazobactamMarcelo UGNo ratings yet
- MP3127 LT 3 210 FlygtDocument7 pagesMP3127 LT 3 210 Flygtİlge Cem TarımcıoğluNo ratings yet
- A M 01 - SpecifikacijeDocument7 pagesA M 01 - SpecifikacijeAlija KolakovicNo ratings yet
- Area of Composite Figures - Day 2 - HomeworkDocument2 pagesArea of Composite Figures - Day 2 - HomeworkCyan AbaraNo ratings yet
- Oil Pan and Suction TubeDocument2 pagesOil Pan and Suction TubeCalon KayaNo ratings yet
- CBL Interpretation Chart PDFDocument1 pageCBL Interpretation Chart PDFReza Alfiano100% (1)
- Pengeluaran Obat Blud Harian Juli 2023Document9 pagesPengeluaran Obat Blud Harian Juli 2023puskesmasmargasatuNo ratings yet
- iK60N iC60N-iC60H-iC60L C120Document11 pagesiK60N iC60N-iC60H-iC60L C120Long Nguyễn Đặng ThànhNo ratings yet
- Enzymatic Purification of GlucomannanDocument12 pagesEnzymatic Purification of GlucomannannaufalrayanNo ratings yet
- Penghitungan Obat Used Syringe PumpDocument31 pagesPenghitungan Obat Used Syringe PumpFregiYuandiNo ratings yet
- Preparation of Buffer Solutions - Pharmaceutical GuidelinesDocument4 pagesPreparation of Buffer Solutions - Pharmaceutical Guidelinesahmed samyNo ratings yet
- Exercise 1Document27 pagesExercise 1Farah Adibah100% (1)
- 2.6 Dilution and ConcentrationDocument12 pages2.6 Dilution and ConcentrationSydney KombeNo ratings yet
- International Journal of PharmaceuticsDocument17 pagesInternational Journal of Pharmaceuticsdavin otooleNo ratings yet
- Product List - ShefaDocument22 pagesProduct List - Shefatarique1189040No ratings yet
- Open Access: Eurasian Journal of Analytical Chemistry ISSN: 1306-3057 2017 12 (7) :987-1000 DOI: 10.12973/ejac.2017.00227aDocument14 pagesOpen Access: Eurasian Journal of Analytical Chemistry ISSN: 1306-3057 2017 12 (7) :987-1000 DOI: 10.12973/ejac.2017.00227aAbhijith AjithNo ratings yet
- PHS 3601 ST Johns University Homework PDocument10 pagesPHS 3601 ST Johns University Homework PHayatfedlumohammedNo ratings yet
- Standardization of Alcohol Calculations in ResearchDocument12 pagesStandardization of Alcohol Calculations in ResearchSava Vijak100% (1)
- Thermo Fisher Scientific 2015 - 16 Chem&GlasswaresDocument152 pagesThermo Fisher Scientific 2015 - 16 Chem&Glasswaresbhusan gurungNo ratings yet
- International Journal of PharmaceuticsDocument8 pagesInternational Journal of PharmaceuticsTrupti Powar WadkarNo ratings yet
- Codex Stan 167 Salted Fish and Dried Salted Fish of The GadidaeDocument10 pagesCodex Stan 167 Salted Fish and Dried Salted Fish of The GadidaeJocilene DantasNo ratings yet
- Lab 7Document3 pagesLab 7Muhd IrfanNo ratings yet
- European Patent Application: Trace Element SolutionDocument15 pagesEuropean Patent Application: Trace Element SolutionMaryam JafariNo ratings yet
- Volumetric Analysis - TaggedDocument11 pagesVolumetric Analysis - TaggedAakash IyengarNo ratings yet
- DOGS TableDocument12 pagesDOGS TableUna CharismaNo ratings yet
- British Pharmacopoeia 2016 Volume 4Document790 pagesBritish Pharmacopoeia 2016 Volume 4Patara ChuparaNo ratings yet
- Liquid Phase Oxidation of Toluene To Benzaldehyde by Air - PDF 1984Document4 pagesLiquid Phase Oxidation of Toluene To Benzaldehyde by Air - PDF 1984Oana VasileNo ratings yet
- Phytochemicals, Antioxidant Activity and Phenolic Profiling of Diplocyclos Palmatus (L.) C. JefferyDocument6 pagesPhytochemicals, Antioxidant Activity and Phenolic Profiling of Diplocyclos Palmatus (L.) C. JefferyJovanJiEunMiNo ratings yet
- Phytochemical Screening of Artabotrys Crassifolius Hook.F. & Thomson (Annonaceae Juss.)Document4 pagesPhytochemical Screening of Artabotrys Crassifolius Hook.F. & Thomson (Annonaceae Juss.)DiarNo ratings yet
- PINK PACOP PHARM CALCULATIONS Answer KeyDocument32 pagesPINK PACOP PHARM CALCULATIONS Answer Keyesther samonteNo ratings yet
- Antibiotics Preparation Chart - ADTDocument8 pagesAntibiotics Preparation Chart - ADTMauricio Alejandro Andino MolinaNo ratings yet
- Chap 04 - Calculations Used in Analytical Chemistry 08Document11 pagesChap 04 - Calculations Used in Analytical Chemistry 08Rashid KanetsaNo ratings yet
- The Carbon Transfer ProcessDocument30 pagesThe Carbon Transfer ProcessuimNo ratings yet
- Chemistry 353, Laboratory 2aDocument4 pagesChemistry 353, Laboratory 2a020101197296No ratings yet
- Flow Injection Spectrophotometric Determination of Acetylsalicylic Acid in Tablets After On-Line Microwave-Assisted Alkaline HydrolysisDocument5 pagesFlow Injection Spectrophotometric Determination of Acetylsalicylic Acid in Tablets After On-Line Microwave-Assisted Alkaline HydrolysisrteofiloNo ratings yet
- Simple Spectrophotometric Method For The Determination of Aescin From Aesculus HippocastanumDocument4 pagesSimple Spectrophotometric Method For The Determination of Aescin From Aesculus Hippocastanumđảm bảo chất lượng thephacoNo ratings yet
- Calculations: Weighing & Measuring Aliquot MethodDocument17 pagesCalculations: Weighing & Measuring Aliquot MethodDanielle Dela PenaNo ratings yet
- Viscotek A-Columns For Aqueous Gpc/Sec User Manual: MAN0498-02-EN-00 October 2015Document8 pagesViscotek A-Columns For Aqueous Gpc/Sec User Manual: MAN0498-02-EN-00 October 2015Heather FlemingNo ratings yet
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (3)
- Meltdown: Nuclear disaster and the human cost of going criticalFrom EverandMeltdown: Nuclear disaster and the human cost of going criticalRating: 5 out of 5 stars5/5 (5)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsFrom EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNo ratings yet
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- Bioplastics: A Home Inventors HandbookFrom EverandBioplastics: A Home Inventors HandbookRating: 4 out of 5 stars4/5 (2)
- Phase Equilibria in Chemical EngineeringFrom EverandPhase Equilibria in Chemical EngineeringRating: 4 out of 5 stars4/5 (11)
- AP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeFrom EverandAP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeRating: 5 out of 5 stars5/5 (1)
- A Perfect Red: Empire, Espionage, and the Quest for the Color of DesireFrom EverandA Perfect Red: Empire, Espionage, and the Quest for the Color of DesireRating: 4 out of 5 stars4/5 (129)
- Transformer: The Deep Chemistry of Life and DeathFrom EverandTransformer: The Deep Chemistry of Life and DeathRating: 4.5 out of 5 stars4.5/5 (13)