Professional Documents
Culture Documents
Ascorbic Acid CoA May 22 (1) - 1
Uploaded by
Studley JupiterOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ascorbic Acid CoA May 22 (1) - 1
Uploaded by
Studley JupiterCopyright:
Available Formats
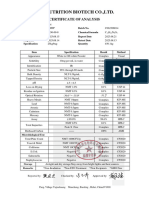
Arndale Ingredients
Nutritional, Pharmaceutical, Food & Beverage Ingredients
CERTIFICATE OF ANALYSIS
Ascorbic Acid
Batch No: 190122026
Manufacturing Date: 4/5/2019
Expiry Date: 3/5/2022
Analysis Standard: BP2016/USP39/EP8.8/FCC10/E300
TEST SPECIFICATION RESULT
Characteristics White Crystalline Powder Pass
Identification Positive Reaction Positive Reaction
Melting Point 190°C 190.4°C
PH 2.4-2.8 (2% Solution) 2.41
2.1-2.6 (5% Solution) 2.33
Clarity of Solution Clear Clear
Colour of Solution BY7max BY7max
Copper 5ppm max 5ppm max
Heavy Metals 10ppm max 10ppm max
Lead 2ppm max 2ppm max
Iron 2ppm max 2ppm max
Mercury 0.1ppm max 0.1ppm max
Arsenic 1ppm max 1ppm max
Cadmium 1ppm max 1ppm max
Zinc 0.25ppm max 0.25ppm max
Loss of Drying ≤0.4% ≤0.4%
Oxalic Acid 0.2% max 0.2% max
Sulphate Ash 0.1% max 0.1% max
Related Impurity C 0.15% max 0.15% max
Substances Impurity D 0.15% max 0.15% max
Unspecified 0.1% max 0.1% max
Total 0.2% max 0.2% max
Impurity E ≤ 0.2% <0.2%
Specific Optical Rotation +20.5° - +21.5° +21.16°
Residual Solvents Pass Pass
Assay 99.0%-100.5% 99.78%
Total Plate Count 100 cfu/g max
E-coli Negative
Yeast and Mould 100 cfu/g max
Pseudomonas Negative
Staphylococcus Negative
Salmonella Negative
Conclusion Conforms to BP2016/USP39/EP8.8/FCC10/E300
As provided by our supplier
Arndale Ingredients UK
Unit D
Western Approach Industrial Estate
Western Approach
South Shields
Tyne and Wear
NE33 5NN
United Kingdom
Email: sales@arndale.co.uk
You might also like
- ASTM Complete Set (Print)Document3 pagesASTM Complete Set (Print)irmuhidin50% (2)
- BS en 515-1993Document22 pagesBS en 515-1993isuru samaranayake100% (1)
- Dan Gelbart Coursenotes2Document23 pagesDan Gelbart Coursenotes2zorkerNo ratings yet
- Project Profile On Soap and Detergent FactoryDocument33 pagesProject Profile On Soap and Detergent Factorysalman92% (13)
- Coa 3220141170Document1 pageCoa 3220141170Andres FarfanNo ratings yet
- 18mt COA 2210142035Document1 page18mt COA 2210142035nadia ARJDALNo ratings yet
- Spec (BP, EP, USP, E300, FCC) - Vitamin C Ex Ningxia QiyuangDocument2 pagesSpec (BP, EP, USP, E300, FCC) - Vitamin C Ex Ningxia QiyuangPaulo Roberto Baggio MoreiraNo ratings yet
- Shandong Luwei Pharmaceutical Co.,Ltd.: Shuangfeng Industrial Park, Zichuan District, Zibo City, Shandong, ChinaDocument1 pageShandong Luwei Pharmaceutical Co.,Ltd.: Shuangfeng Industrial Park, Zichuan District, Zibo City, Shandong, ChinaSouheila MniNo ratings yet
- COA of Calcium D Pantothente - RevisedDocument1 pageCOA of Calcium D Pantothente - RevisedJose.SuarezNo ratings yet
- Ascorbic Acid-Shandong LuweiDocument1 pageAscorbic Acid-Shandong LuweiSai Kiran PalikaNo ratings yet
- MSDS Vitamin C (E300)Document6 pagesMSDS Vitamin C (E300)Niken Tri WahyuningsihNo ratings yet
- Coa - Cocoa Ah01 OctubreDocument2 pagesCoa - Cocoa Ah01 OctubreChristopher CaizaNo ratings yet
- 20220829无水柠檬酸(英文、无图) 09534070492Document1 page20220829无水柠檬酸(英文、无图) 09534070492JoseNo ratings yet
- Certificate of Analysis: Shanghai Hy-Sailing Chemical Tech. Co.,LTDDocument1 pageCertificate of Analysis: Shanghai Hy-Sailing Chemical Tech. Co.,LTDrocio oteroNo ratings yet
- Certificado de Analisis: - Certificate of Analysis As Received From Our Supplier - Productos Químicos Gonmisol S.A.Document2 pagesCertificado de Analisis: - Certificate of Analysis As Received From Our Supplier - Productos Químicos Gonmisol S.A.pervaz anwerNo ratings yet
- CoA Sucralose - Supplier Tokped (Shandong Kanbo)Document1 pageCoA Sucralose - Supplier Tokped (Shandong Kanbo)Tantriyani GunadyNo ratings yet
- Certificate of Analysis: Hubei Xingfa Chemicals Group Co., LTDDocument1 pageCertificate of Analysis: Hubei Xingfa Chemicals Group Co., LTDSpecialty Chemicals100% (1)
- Coa Polivinilpirrolidona K-90 (PVP K-90) Lote 20221019Document1 pageCoa Polivinilpirrolidona K-90 (PVP K-90) Lote 20221019Ives AlbarracinNo ratings yet
- Hoodia Gordonii Extract COADocument1 pageHoodia Gordonii Extract COAStefani StefaniNo ratings yet
- Bengel MBF-2000Document1 pageBengel MBF-2000arron_jacklinNo ratings yet
- CoA HYDROXYLAMINE HYDROCHLORIDE Loba ChemieDocument1 pageCoA HYDROXYLAMINE HYDROCHLORIDE Loba ChemieimamaptNo ratings yet
- CAA CAM specification - BP USP FCC8 E330 - 副本Document2 pagesCAA CAM specification - BP USP FCC8 E330 - 副本xuân ba caoNo ratings yet
- FT Shandong Luwei Pharmaceutical Rev 120421Document1 pageFT Shandong Luwei Pharmaceutical Rev 120421Control calidadNo ratings yet
- COA Pelargonium Sidoides Extract4-1Document1 pageCOA Pelargonium Sidoides Extract4-1Bilal MasoodNo ratings yet
- 08 Coa Apl-Clp-01285-I-23Document1 page08 Coa Apl-Clp-01285-I-23bpharmbaNo ratings yet
- Ginseng Leaf ExtractDocument1 pageGinseng Leaf Extractyousfinadjah5No ratings yet
- Specification Aloe Vera Gel Freeze Dried PowderDocument1 pageSpecification Aloe Vera Gel Freeze Dried PowderFernando DuqueNo ratings yet
- Acesulfame K - 203207843Document1 pageAcesulfame K - 203207843Kevin RiveraNo ratings yet
- FT Goma XantanDocument1 pageFT Goma XantanMaria CalderonNo ratings yet
- 5-HTP CoaDocument1 page5-HTP Coawillyvh99No ratings yet
- KT - Dextrose Monohydrate Food GradeDocument1 pageKT - Dextrose Monohydrate Food GradeAngel RamirezNo ratings yet
- Certificate of AnalysisDocument1 pageCertificate of AnalysisfitaNo ratings yet
- Coa Alpha 2108n21Document1 pageCoa Alpha 2108n21dwiyulianto28No ratings yet
- How To Treat Refinery Gases To Recover Valuable GasesDocument21 pagesHow To Treat Refinery Gases To Recover Valuable GasesMădălina GrigorescuNo ratings yet
- Paramters Removal Efficiency-Pilot UnitDocument6 pagesParamters Removal Efficiency-Pilot UnitRoger T SomundohNo ratings yet
- Vitamin CDocument2 pagesVitamin CJimmy Bayu WibowoNo ratings yet
- Norms LECTUREDocument5 pagesNorms LECTUREKAIVALYA TIWATNENo ratings yet
- COA of Riboflavin 5 - Sodium Phosphate 23120803Document2 pagesCOA of Riboflavin 5 - Sodium Phosphate 23120803Dwi Satria PutraNo ratings yet
- Citric AcidDocument3 pagesCitric Acidrizka wahyuni helsinNo ratings yet
- Xanthan Gum 200meshDocument1 pageXanthan Gum 200meshRobert ConwayNo ratings yet
- CoA of EgcgDocument1 pageCoA of EgcgMirna Candra RNo ratings yet
- COA-Carnitine HCL-HengtaiDocument1 pageCOA-Carnitine HCL-Hengtaichurch.hrgNo ratings yet
- dsxg02 Ziboxan f200Document1 pagedsxg02 Ziboxan f200thái học “hochaha”No ratings yet
- International Standards For Biodiesel and South African Test MethodsDocument1 pageInternational Standards For Biodiesel and South African Test MethodsskosanaNo ratings yet
- Cement ComparisonsDocument1 pageCement ComparisonsShane PhillipsNo ratings yet
- RSL SpecificationDocument2 pagesRSL Specificationmeli meliNo ratings yet
- Prosedur K3Document39 pagesProsedur K3reza dian humkiNo ratings yet
- Acetone For Analysis, Reag. ACS, Reag. ISO, Reag. Ph. EurDocument2 pagesAcetone For Analysis, Reag. ACS, Reag. ISO, Reag. Ph. EurFethi FatnassiNo ratings yet
- Glycerin Grade Comparison ChartDocument2 pagesGlycerin Grade Comparison Chartisraa helalNo ratings yet
- GIẤY CHỨNG NHẬN CHẤT LƯỢNG SẢN PHẨM 3Document4 pagesGIẤY CHỨNG NHẬN CHẤT LƯỢNG SẢN PHẨM 3Uyên ĐặngNo ratings yet
- TSD Stevia Extract (G)Document1 pageTSD Stevia Extract (G)FranciscaGarcésNo ratings yet
- Calcipur 2-OG SpecDocument1 pageCalcipur 2-OG SpecBenoit BLANCHETNo ratings yet
- Propylene Glycol Pharma Grade FTDocument1 pagePropylene Glycol Pharma Grade FTDiego GuzmánNo ratings yet
- Snowhite 80Document1 pageSnowhite 80Mateus PachecoNo ratings yet
- Lycopene 5%oilDocument1 pageLycopene 5%oilNguyễn Ngọc NamNo ratings yet
- COA of Bromelain 1200 GDUDocument1 pageCOA of Bromelain 1200 GDUEndah WulandariNo ratings yet
- Certificate of Analysis Sodium Bicarbonate, Usp Grade #1: N1181811-1 06/30/18 Manufacturing Date: LOT NUMBER (Packaged)Document1 pageCertificate of Analysis Sodium Bicarbonate, Usp Grade #1: N1181811-1 06/30/18 Manufacturing Date: LOT NUMBER (Packaged)Carlos Awo OsaureNo ratings yet
- Finished Product Formula & Specs: Dal Animal FeedDocument1 pageFinished Product Formula & Specs: Dal Animal Feedanas hussainNo ratings yet
- LW Trade - A4 - 2023 - 08 - 16Document12 pagesLW Trade - A4 - 2023 - 08 - 169229113No ratings yet
- TDS ApDocument1 pageTDS ApYole IstaNo ratings yet
- Coa 76643Document1 pageCoa 76643Rabah ABBASNo ratings yet
- HNC Pyro ATM Final ProductsDocument2 pagesHNC Pyro ATM Final Productssales5000No ratings yet
- CompleximetryDocument29 pagesCompleximetrychamp delacruzNo ratings yet
- 1119HCDS205v2 Zinador 35LDocument6 pages1119HCDS205v2 Zinador 35LUtpalNo ratings yet
- FOSFA List of Acceptable Previous Cargoes With Flowchart Nov 2022Document4 pagesFOSFA List of Acceptable Previous Cargoes With Flowchart Nov 2022jesusNo ratings yet
- Protection Tubes and Thermowells: Thermowell Materials Selection GuideDocument3 pagesProtection Tubes and Thermowells: Thermowell Materials Selection GuideJp NairNo ratings yet
- Us5098778 PDFDocument7 pagesUs5098778 PDFAl Saraaf MohammedNo ratings yet
- Why Is There A Need of Dosage FormDocument2 pagesWhy Is There A Need of Dosage FormArchie CabacheteNo ratings yet
- AODA Work Sheet ExamsDocument11 pagesAODA Work Sheet Examsrachel2904No ratings yet
- NSE Stage 1 2016 17 Solution NSEC Code C322 v1Document29 pagesNSE Stage 1 2016 17 Solution NSEC Code C322 v1Chirayu VermaNo ratings yet
- Chem 9Document4 pagesChem 9Emmanuel PlazaNo ratings yet
- Periodic Table of Elements: Scientists Have Identified 90 Naturally Occurring Elements, and Created About 28 OthersDocument54 pagesPeriodic Table of Elements: Scientists Have Identified 90 Naturally Occurring Elements, and Created About 28 OthersTrixieCamposanoNo ratings yet
- Ixora Chinensis Flower Extract Natural IndicatorDocument6 pagesIxora Chinensis Flower Extract Natural Indicatorapi-19918842No ratings yet
- Name Reaction Reagent Assignment PDFDocument21 pagesName Reaction Reagent Assignment PDFSandipan SahaNo ratings yet
- Leader in Superabrasive Finishing Systems: Pad Applications We Offer A Full Line of Hyprez® ProductsDocument2 pagesLeader in Superabrasive Finishing Systems: Pad Applications We Offer A Full Line of Hyprez® Productsarmin heidariNo ratings yet
- Cadmium Free Brazing AlloysDocument4 pagesCadmium Free Brazing AlloysNileshhkNo ratings yet
- Astm F2999-19Document8 pagesAstm F2999-19nickhoNo ratings yet
- 8th Metal and Non MetalDocument8 pages8th Metal and Non MetalsubrotokumarmohantaNo ratings yet
- Eggert, G. y Schmutzler B. (Ed.) - Archaeological Iron Conservation. 2010Document98 pagesEggert, G. y Schmutzler B. (Ed.) - Archaeological Iron Conservation. 2010Trinidad Pasíes Arqueología-Conservación100% (2)
- Pharmaceutical Statistics NEW: Pharmaceutics / Industrial Pharmacy / Pharmaceutical TechnologyDocument8 pagesPharmaceutical Statistics NEW: Pharmaceutics / Industrial Pharmacy / Pharmaceutical TechnologyIlhan KhanNo ratings yet
- Solsperse Hyperdispersants Overview - 19-179712Document2 pagesSolsperse Hyperdispersants Overview - 19-179712Jose E BatistaNo ratings yet
- A02 037Document21 pagesA02 037jaimeNo ratings yet
- Phyto ManjakaniDocument19 pagesPhyto ManjakaniThirukumaren JeyasagarenNo ratings yet
- Kitchen Chemistry Powerpoint PresentationDocument17 pagesKitchen Chemistry Powerpoint PresentationAnika Anjum SaraNo ratings yet
- Wrobleski 2004Document7 pagesWrobleski 2004Saurav PaulNo ratings yet
- Venpure Chemo PDFDocument4 pagesVenpure Chemo PDFFernando J. Correa DelgadoNo ratings yet
- Chapter 12. Practice Problems Part I. Do As IndicatedDocument2 pagesChapter 12. Practice Problems Part I. Do As IndicatedAlwyn Dave AmbataliNo ratings yet
- USP-NF Cefotaxime SodiumDocument6 pagesUSP-NF Cefotaxime SodiumCongluanNo ratings yet