Professional Documents
Culture Documents
18mt COA 2210142035
Uploaded by
nadia ARJDAL0 ratings0% found this document useful (0 votes)
29 views1 pageOriginal Title
18mt COA 2210142035 (1)
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
29 views1 page18mt COA 2210142035
Uploaded by
nadia ARJDALCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
LUWEI PHARMACEUTICAL GROUP CO.,LTD.
SHUANGFENG INDUSTRIAL PARK,ZICHUAN DISTRICT,ZIBO CIY,SHANDONG,CHINA
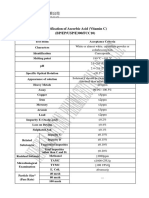
CERTIFICATE OF ANALYSIS
LWPC-2C1-21-11-037
Commodity: ASCORBIC ACID Date: 22.DEC.2021
Batch No.: 2210142035 Manufacture Date: NOV.2021
Quantity: 18000kgs Expiry Date: OCT.2024
Analysis Contents Analysis Standard Analysis Results
White or almost White
Characteristics Pass
crystals Crystalline Powder
Identification Positive Reaction Positive
Melting Point 190.9℃
About 190℃
PH(5% aqueous solution) 2.1-2.6 2.36
Clarity Of Solution Clear Clear
Colour Of Solution ≤BY7 <BY7
Copper ≤5ppm <5ppm
Heavy Metals ≤10ppm <10ppm
Mercury <0.1mg/kg <0.1mg/kg
Lead <2mg/kg <2mg/kg
Arsenic ≤2ppm <2ppm
Oxalic Acid ≤0.2% <0.2%
Iron ≤2ppm <2ppm
Impurity E ≤0.2% <0.2%
Loss on Drying ≤0.4% 0.05%
Sulphate Ash(Residue On Ignition) ≤0.1% <0.1%
Specific Optical Rotation +20.5°–+21.5° +21.16°
Organic Volatile Impurities Pass Pass
99.0%-100.5%
Assay 99.0% -100.5% 99.72%
Total plate count ≤1000cfu/g <100cfu/g
Yeasts & molds ≤100cfu/g <10cfu/g
The Above-Mentioned Product Conforms To
Conclusion: BP2019/USP42
You might also like
- TPCHWG Compositionvol2Document114 pagesTPCHWG Compositionvol2sgutierNo ratings yet
- Activity No 7 - Ethyl AlcoholDocument2 pagesActivity No 7 - Ethyl Alcoholpharmaebooks67% (3)
- ChemistryDocument11 pagesChemistryJoniele Angelo Anin100% (1)
- Coa 3220141170Document1 pageCoa 3220141170Andres FarfanNo ratings yet
- Ascorbic Acid-Shandong LuweiDocument1 pageAscorbic Acid-Shandong LuweiSai Kiran PalikaNo ratings yet
- Shandong Luwei Pharmaceutical Co.,Ltd.: Shuangfeng Industrial Park, Zichuan District, Zibo City, Shandong, ChinaDocument1 pageShandong Luwei Pharmaceutical Co.,Ltd.: Shuangfeng Industrial Park, Zichuan District, Zibo City, Shandong, ChinaSouheila MniNo ratings yet
- Vitamin CDocument2 pagesVitamin CJimmy Bayu WibowoNo ratings yet
- 1 COA of Carbopol 940 PDFDocument1 page1 COA of Carbopol 940 PDFHayk HayrapetyanNo ratings yet
- KT - Dextrose Monohydrate Food GradeDocument1 pageKT - Dextrose Monohydrate Food GradeAngel RamirezNo ratings yet
- Coa Polivinilpirrolidona K-90 (PVP K-90) Lote 20221019Document1 pageCoa Polivinilpirrolidona K-90 (PVP K-90) Lote 20221019Ives AlbarracinNo ratings yet
- Ascorbic Acid CoA May 22 (1) - 1Document2 pagesAscorbic Acid CoA May 22 (1) - 1Studley JupiterNo ratings yet
- Acido Benzoico Food Grade - Lot 20201026 - TianjiaDocument1 pageAcido Benzoico Food Grade - Lot 20201026 - TianjiaArmando GarciaNo ratings yet
- Vitamin C Specification GuideDocument2 pagesVitamin C Specification GuidePaulo Roberto Baggio MoreiraNo ratings yet
- Citric Acid Mono - Coa - Weifang (Oct. 2021) Batch No. 1mt2110002Document1 pageCitric Acid Mono - Coa - Weifang (Oct. 2021) Batch No. 1mt2110002Huynh DanhNo ratings yet
- Acesulfame K - 203207843Document1 pageAcesulfame K - 203207843Kevin RiveraNo ratings yet
- West China HengRui Biotech Calcium D-Pantothenate Certificate of AnalysisDocument1 pageWest China HengRui Biotech Calcium D-Pantothenate Certificate of AnalysisJose.SuarezNo ratings yet
- COA-Carnitine HCL-HengtaiDocument1 pageCOA-Carnitine HCL-Hengtaichurch.hrgNo ratings yet
- Certificate of Analysis: Shanghai Hy-Sailing Chemical Tech. Co.,LTDDocument1 pageCertificate of Analysis: Shanghai Hy-Sailing Chemical Tech. Co.,LTDrocio oteroNo ratings yet
- Certificate of Analysis: Hubei Xingfa Chemicals Group Co., LTDDocument1 pageCertificate of Analysis: Hubei Xingfa Chemicals Group Co., LTDSpecialty Chemicals100% (1)
- 2017 - Product Specification - RZBC (JUXIAN) - CAA PDFDocument1 page2017 - Product Specification - RZBC (JUXIAN) - CAA PDFediasianagriNo ratings yet
- 2017 - Product Specification - RZBC (JUXIAN) - CAADocument1 page2017 - Product Specification - RZBC (JUXIAN) - CAAediasianagri100% (1)
- Citric Acid Anhydrate SpesifikasiDocument1 pageCitric Acid Anhydrate SpesifikasicinnamaldehidNo ratings yet
- Citric Acid Anhydrous RZBC (JUXIAN) CO., LTD PDFDocument2 pagesCitric Acid Anhydrous RZBC (JUXIAN) CO., LTD PDFOzzymandias100% (1)
- CoA of EgcgDocument1 pageCoA of EgcgMirna Candra RNo ratings yet
- COA Citric Acid M202506Document1 pageCOA Citric Acid M202506nadia ARJDALNo ratings yet
- Coa STPP XingfaDocument1 pageCoa STPP XingfaNha TranNo ratings yet
- Coa-Stpp-W032022-Chongqing (21.04.22)Document1 pageCoa-Stpp-W032022-Chongqing (21.04.22)Nha TranNo ratings yet
- Coa - Cocoa Ah01 OctubreDocument2 pagesCoa - Cocoa Ah01 OctubreChristopher CaizaNo ratings yet
- Certificate of Analysis: 17α-Hydroxy Progesterone AcetateDocument2 pagesCertificate of Analysis: 17α-Hydroxy Progesterone Acetatewindli2012No ratings yet
- SAPP FG - SpecificationDocument1 pageSAPP FG - SpecificationNha TranNo ratings yet
- FT Shandong Luwei Pharmaceutical Rev 120421Document1 pageFT Shandong Luwei Pharmaceutical Rev 120421Control calidadNo ratings yet
- Wuxi R and D Chemical Co.,Ltd.: 1606 Hodo Int'L Plaza, No.531 Zhongshan Road, Wuxi, Jiangsu, ChinaDocument1 pageWuxi R and D Chemical Co.,Ltd.: 1606 Hodo Int'L Plaza, No.531 Zhongshan Road, Wuxi, Jiangsu, Chinanadia ARJDALNo ratings yet
- VC Ascorbic Acid 100 Mesh 90%: Coversheet For Certificate of AnalysisDocument3 pagesVC Ascorbic Acid 100 Mesh 90%: Coversheet For Certificate of AnalysisNicole Paredes Del AguilaNo ratings yet
- 20220829无水柠檬酸(英文、无图) 09534070492Document1 page20220829无水柠檬酸(英文、无图) 09534070492JoseNo ratings yet
- Toyond Industry Limited: Certificate of AnalysisDocument2 pagesToyond Industry Limited: Certificate of AnalysisQf Jhon DonadoNo ratings yet
- Certificado de Analisis: - Certificate of Analysis As Received From Our Supplier - Productos Químicos Gonmisol S.A.Document2 pagesCertificado de Analisis: - Certificate of Analysis As Received From Our Supplier - Productos Químicos Gonmisol S.A.pervaz anwerNo ratings yet
- CoA HYDROXYLAMINE HYDROCHLORIDE Loba ChemieDocument1 pageCoA HYDROXYLAMINE HYDROCHLORIDE Loba ChemieimamaptNo ratings yet
- CoA Sucralose - Supplier Tokped (Shandong Kanbo)Document1 pageCoA Sucralose - Supplier Tokped (Shandong Kanbo)Tantriyani GunadyNo ratings yet
- 220601-Shandong KundaDocument1 page220601-Shandong KundaGregorius SimarmataNo ratings yet
- Hoodia Gordonii Extract Certificate of AnalysisDocument1 pageHoodia Gordonii Extract Certificate of AnalysisStefani StefaniNo ratings yet
- PS 005 - AyuFlexDocument1 pagePS 005 - AyuFlexLuis Castro XtrmNo ratings yet
- Coa 76643Document1 pageCoa 76643Rabah ABBASNo ratings yet
- Coa PDFDocument4 pagesCoa PDFsaidNo ratings yet
- CoA RZBC Citric AcidDocument3 pagesCoA RZBC Citric AcidSofyan TanotoNo ratings yet
- Wuxi R&D Chemical Co. Citric Acid Certificate of AnalysisDocument1 pageWuxi R&D Chemical Co. Citric Acid Certificate of Analysisnadia ARJDALNo ratings yet
- CITRIC ACID AnalysisDocument1 pageCITRIC ACID AnalysisZaryab ArifNo ratings yet
- COA - Sodium Ascorbate Ex DSM Jiangshan PharmaDocument2 pagesCOA - Sodium Ascorbate Ex DSM Jiangshan PharmaIhin SolihinNo ratings yet
- LW Trade_A4_2023_08_16Document12 pagesLW Trade_A4_2023_08_169229113No ratings yet
- Potassium Nitrate Without Anticaking (BP, Ph. Eur.) Pure, Pharma GradeDocument1 pagePotassium Nitrate Without Anticaking (BP, Ph. Eur.) Pure, Pharma GradeMiguel CruzNo ratings yet
- HISTIDINEDocument1 pageHISTIDINEAhmedNo ratings yet
- 1124certificate of AnalysisDocument1 page1124certificate of AnalysisTutorial Bahasa IndonesiaNo ratings yet
- 1 Coa-DcpDocument1 page1 Coa-DcpjivaorganicNo ratings yet
- COA OF MENTHOL 薄荷脑Document1 pageCOA OF MENTHOL 薄荷脑Gioacchino MondelloNo ratings yet
- Certificate of Analysis: Characteristics Specifications Measured ValuesDocument2 pagesCertificate of Analysis: Characteristics Specifications Measured ValuesCosmin CostiNo ratings yet
- Coa Alpha 2108n21Document1 pageCoa Alpha 2108n21dwiyulianto28No ratings yet
- Xanthan Gum 200meshDocument1 pageXanthan Gum 200meshRobert ConwayNo ratings yet
- COA - Sulphuric AcidDocument2 pagesCOA - Sulphuric AcidMechem EurofinsNo ratings yet
- Product Analysis Certificate: Propanol-2 (Iso-Propanol) A.RDocument1 pageProduct Analysis Certificate: Propanol-2 (Iso-Propanol) A.RAMMARNo ratings yet
- COA -Ascorbic Acid,B# 1101070022-2Document1 pageCOA -Ascorbic Acid,B# 1101070022-2Aboudeh FarranNo ratings yet
- Lampiran III - CoA NSF-04Document1 pageLampiran III - CoA NSF-04Ferdian Iwank IriyantoNo ratings yet
- D-RIBOSE COADocument1 pageD-RIBOSE COAwillyvh99No ratings yet
- Atmospheric Chemical Compounds: Sources, Occurrence and BioassayFrom EverandAtmospheric Chemical Compounds: Sources, Occurrence and BioassayNo ratings yet
- TDS - MPGDocument1 pageTDS - MPGnadia ARJDALNo ratings yet
- COA Citric Acid M202506Document1 pageCOA Citric Acid M202506nadia ARJDALNo ratings yet
- Wuxi R&D Chemical Co. Citric Acid Certificate of AnalysisDocument1 pageWuxi R&D Chemical Co. Citric Acid Certificate of Analysisnadia ARJDALNo ratings yet
- Safety Data Sheet: (According To Regulation (EC) No. 1907/2006)Document9 pagesSafety Data Sheet: (According To Regulation (EC) No. 1907/2006)nadia ARJDALNo ratings yet
- Wuxi R and D Chemical Co.,Ltd.: 1606 Hodo Int'L Plaza, No.531 Zhongshan Road, Wuxi, Jiangsu, ChinaDocument1 pageWuxi R and D Chemical Co.,Ltd.: 1606 Hodo Int'L Plaza, No.531 Zhongshan Road, Wuxi, Jiangsu, Chinanadia ARJDALNo ratings yet
- COA-sodium Benzoate GranularDocument1 pageCOA-sodium Benzoate Granularnadia ARJDALNo ratings yet
- Metals and Non Metals Class 10Document26 pagesMetals and Non Metals Class 10mohammedahsanxxNo ratings yet
- Wako Product UpdateDocument20 pagesWako Product UpdatetoanvmpetrologxNo ratings yet
- CVFGFHGDocument25 pagesCVFGFHGMary Grace VelitarioNo ratings yet
- Ammonium Copper (II) Sulphate PreparationDocument5 pagesAmmonium Copper (II) Sulphate PreparationMpilo ManyoniNo ratings yet
- Organic Compounds Functional GroupsDocument2 pagesOrganic Compounds Functional Groupskendall knightNo ratings yet
- Lel 2Document2 pagesLel 2Hossam A.MoneimNo ratings yet
- Phytochemical Screening and Identification of Phenolic Compounds in MallowDocument5 pagesPhytochemical Screening and Identification of Phenolic Compounds in MallowDinh DungNo ratings yet
- Wilkinson's Catalyst Mechanism and UsesDocument10 pagesWilkinson's Catalyst Mechanism and UsesomansuNo ratings yet
- Ftalilsulfatiazol. Farmacopea Europea 8 Ed. Vol 2-1529 PDFDocument1 pageFtalilsulfatiazol. Farmacopea Europea 8 Ed. Vol 2-1529 PDFmariacalasinfoNo ratings yet
- COA of L - GlutathioneDocument1 pageCOA of L - GlutathionePan EmmaNo ratings yet
- Process for Electroplating Rhodium to Produce Black or Blue ColorDocument6 pagesProcess for Electroplating Rhodium to Produce Black or Blue ColorDhandapani PNo ratings yet
- Treating Phosphate Fertilizer Plant Waste Water with Reverse OsmosisDocument12 pagesTreating Phosphate Fertilizer Plant Waste Water with Reverse OsmosisCarlosNo ratings yet
- Pe312-Natural Gas Engineering S&DDocument33 pagesPe312-Natural Gas Engineering S&DMalugu JohnNo ratings yet
- Elementis Personal Care Formulary Dec 2013Document8 pagesElementis Personal Care Formulary Dec 2013Rina ABDALINo ratings yet
- Buffers: Maintaining pH EquilibriumDocument62 pagesBuffers: Maintaining pH EquilibriumSara FatimaNo ratings yet
- Biodegradation of Textile WastewaterDocument1 pageBiodegradation of Textile WastewaterJorge Froilan GonzalezNo ratings yet
- Reaction of Alkenes and Alkynes For StudentsDocument53 pagesReaction of Alkenes and Alkynes For StudentsGlen MangaliNo ratings yet
- Norma CipwDocument32 pagesNorma CipwAlonso CeliNo ratings yet
- Basic Wash Terms TutorialDocument28 pagesBasic Wash Terms TutorialQiao XiaokangNo ratings yet
- 12th Board Sprint-Amines (15.12.2020)Document62 pages12th Board Sprint-Amines (15.12.2020)Harsh ShahNo ratings yet
- Chemistry III Formulas and NamingDocument2 pagesChemistry III Formulas and NamingAngela CatainaNo ratings yet
- Sri Kailash Chemicals ManufacturerDocument13 pagesSri Kailash Chemicals ManufacturerSumanChowdaryNo ratings yet
- Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced LevelDocument16 pagesCambridge International Examinations Cambridge International Advanced Subsidiary and Advanced LevelSangkari Karuppiah GanesanNo ratings yet
- Comparison of Extraction Different Methods of Sodium Alginate From Brown AlgaDocument5 pagesComparison of Extraction Different Methods of Sodium Alginate From Brown AlgaAnonymous GXvhtBw67TNo ratings yet
- AP Chemistry - Titration Curves of Strong and Weak Acids and BasesDocument5 pagesAP Chemistry - Titration Curves of Strong and Weak Acids and BasesJonathan Chen100% (2)
- Vibrational Studies of Na SO K SO Nahso and Khso Crystals: Azha - Periasamy, S.Muruganand and M.PalaniswamyDocument9 pagesVibrational Studies of Na SO K SO Nahso and Khso Crystals: Azha - Periasamy, S.Muruganand and M.PalaniswamyMelin YohanaNo ratings yet