Professional Documents
Culture Documents
Certificate of Analysis: 17α-Hydroxy Progesterone Acetate
Uploaded by
windli20120 ratings0% found this document useful (0 votes)
37 views2 pages6-Hydroxyprogesterone acetate Unknown impurity Residual Solvents (GC) Methanol Ethanol Dichloromethane Pyridine Acetate acid Assay(HPLC) Conclusion: Analysed by: Date: Chief of QC: Chief of Qa: Date: Complies with In-house quality control procedures.
Original Description:
Original Title
0
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document6-Hydroxyprogesterone acetate Unknown impurity Residual Solvents (GC) Methanol Ethanol Dichloromethane Pyridine Acetate acid Assay(HPLC) Conclusion: Analysed by: Date: Chief of QC: Chief of Qa: Date: Complies with In-house quality control procedures.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
37 views2 pagesCertificate of Analysis: 17α-Hydroxy Progesterone Acetate
Uploaded by
windli20126-Hydroxyprogesterone acetate Unknown impurity Residual Solvents (GC) Methanol Ethanol Dichloromethane Pyridine Acetate acid Assay(HPLC) Conclusion: Analysed by: Date: Chief of QC: Chief of Qa: Date: Complies with In-house quality control procedures.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
You are on page 1of 2
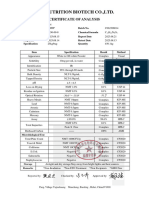
Certificate of Analysis
17α-Hydroxy Progesterone Acetate
Product:
Batch No.: 035-20100501H Mfg. Date: 2010-04-06 Analysis Date: 2010-05-22
Quantity: 10g CAS No.: 302-23-8 Retest Date: 2013-04-05
In-house
Storage: Preserve in tight, light-resistant containers. Standard: Standard
ITEM AND RESULT
Item Specification Result
Ivory-white crystalline Ivory-white crystalline
Appearance substance substance
HPLC
Identification IR Complies
Loss on drying ≤0.5% 0.1%
Specific optical rotation +58°~+62° +59.2°
Sulphated ash ≤0.10% Complies
17α-Hydroxyprogesterone ≤0.5% 0.1%
Chromatographic △6-Hydroxyprogesterone
purity acetate ≤0.1% 0.1%
Unknown impurity ≤0.10% 0.09%
Methanol ≤3000ppm 114 ppm
Ethanol ≤5000ppm 35ppm
Residual Solvents
Dichloromethane ≤600ppm 278 ppm
(GC)
Pyridine ≤200ppm 6ppm
Acetate acid ≤5000ppm 58ppm
Assay(HPLC) 98.0%~102.0% 99.1%
Conclusion: Complies with In-house Standard
Analysed by: Chief of QC: Chief of QA:
Date: Date: Date:
You might also like
- Records Retention Policy and ScheduleDocument7 pagesRecords Retention Policy and Schedulewindli2012No ratings yet
- CQV #2 Like-For-Like Change ProblemsDocument7 pagesCQV #2 Like-For-Like Change Problemswindli2012No ratings yet
- Coa of Bcaa 4-1-1Document1 pageCoa of Bcaa 4-1-1dokterasadNo ratings yet
- Citric Acid Anhydrous RZBC (JUXIAN) CO., LTD PDFDocument2 pagesCitric Acid Anhydrous RZBC (JUXIAN) CO., LTD PDFOzzymandias100% (1)
- Inventarisasi Limbah B3Document22 pagesInventarisasi Limbah B3Pelayanan Medis RSGH100% (3)
- COA-Carnitine HCL-HengtaiDocument1 pageCOA-Carnitine HCL-Hengtaichurch.hrgNo ratings yet
- Coa 3220141170Document1 pageCoa 3220141170Andres FarfanNo ratings yet
- 18mt COA 2210142035Document1 page18mt COA 2210142035nadia ARJDALNo ratings yet
- CoA of EgcgDocument1 pageCoA of EgcgMirna Candra RNo ratings yet
- COA Elacestrant Dihydrochloride Shandongkehui - 20240120221842Document2 pagesCOA Elacestrant Dihydrochloride Shandongkehui - 20240120221842rashidulhasan789No ratings yet
- Citric Acid Anhydrate SpesifikasiDocument1 pageCitric Acid Anhydrate SpesifikasicinnamaldehidNo ratings yet
- 2017 - Product Specification - RZBC (JUXIAN) - CAADocument1 page2017 - Product Specification - RZBC (JUXIAN) - CAAediasianagri100% (1)
- 2017 - Product Specification - RZBC (JUXIAN) - CAA PDFDocument1 page2017 - Product Specification - RZBC (JUXIAN) - CAA PDFediasianagriNo ratings yet
- Xi'an Haoxuan Bio-Tech Co.,Ltd: Certificate of AnylasisDocument1 pageXi'an Haoxuan Bio-Tech Co.,Ltd: Certificate of AnylasisNilsNo ratings yet
- COA OF MENTHOL 薄荷脑Document1 pageCOA OF MENTHOL 薄荷脑Gioacchino MondelloNo ratings yet
- CoA Sucralose - Supplier Tokped (Shandong Kanbo)Document1 pageCoA Sucralose - Supplier Tokped (Shandong Kanbo)Tantriyani GunadyNo ratings yet
- COA Panax Ginseng ExtDocument1 pageCOA Panax Ginseng ExtFerdian Iwank IriyantoNo ratings yet
- Xi'an Haoxuan Bio-Tech Co.,Ltd: Certificate of AnylasisDocument1 pageXi'an Haoxuan Bio-Tech Co.,Ltd: Certificate of AnylasisNilsNo ratings yet
- 1, Water Soluble Ginkgo Biloba ExtractDocument1 page1, Water Soluble Ginkgo Biloba ExtractdanilriosNo ratings yet
- COA of Calcium D Pantothente - RevisedDocument1 pageCOA of Calcium D Pantothente - RevisedJose.SuarezNo ratings yet
- 5-HTP CoaDocument1 page5-HTP Coawillyvh99No ratings yet
- Toyond Industry Limited: Certificate of AnalysisDocument2 pagesToyond Industry Limited: Certificate of AnalysisQf Jhon DonadoNo ratings yet
- Coa 20040202 PDFDocument1 pageCoa 20040202 PDFRisen ChemicalsNo ratings yet
- Sorbitol 70% Non Crystallizing Liquid RM COADocument2 pagesSorbitol 70% Non Crystallizing Liquid RM COAASHOK KUMAR LENKANo ratings yet
- Caffeine Natural Coffee BeanDocument1 pageCaffeine Natural Coffee BeanMayang TariNo ratings yet
- L (+) ORNITINA A3450 - 6d015448Document1 pageL (+) ORNITINA A3450 - 6d015448junio16No ratings yet
- Parameter USP 34 Specification Reagent Plus (C0750) Caffeine Sigma Reference Standard (C1778)Document2 pagesParameter USP 34 Specification Reagent Plus (C0750) Caffeine Sigma Reference Standard (C1778)rushikeshghuleNo ratings yet
- 1 COA of Carbopol 940 PDFDocument1 page1 COA of Carbopol 940 PDFHayk HayrapetyanNo ratings yet
- Ursodeoxycholic Acid Sichuan Xieli Coa (Ep6.0)Document1 pageUrsodeoxycholic Acid Sichuan Xieli Coa (Ep6.0)ciciliaNo ratings yet
- Ethanol Absolute For UV, IR, HPLC: Code: Batch: Product: Issue Date: Retest DateDocument1 pageEthanol Absolute For UV, IR, HPLC: Code: Batch: Product: Issue Date: Retest DateCamilo Andrés MIrandaNo ratings yet
- Stevia GS90% COADocument1 pageStevia GS90% COAFranck BlauNo ratings yet
- Specification Aloe Vera Gel Freeze Dried PowderDocument1 pageSpecification Aloe Vera Gel Freeze Dried PowderFernando DuqueNo ratings yet
- Salicylic Acid 69-72-7 COADocument1 pageSalicylic Acid 69-72-7 COASACO QCNo ratings yet
- Certificate of Analysis: Polysorbate 80Document1 pageCertificate of Analysis: Polysorbate 80Nurul HidayatriNo ratings yet
- Citric Acid Mono - Coa - Weifang (Oct. 2021) Batch No. 1mt2110002Document1 pageCitric Acid Mono - Coa - Weifang (Oct. 2021) Batch No. 1mt2110002Huynh DanhNo ratings yet
- Certificate of Analysis: Name of Product (Leepol - 980) Generic Name Acrylate Co PolymerDocument1 pageCertificate of Analysis: Name of Product (Leepol - 980) Generic Name Acrylate Co Polymerjuan felixNo ratings yet
- Methyl Anthranilate COA PDFDocument1 pageMethyl Anthranilate COA PDFAzkiyah RahmahNo ratings yet
- Vitamin CDocument2 pagesVitamin CJimmy Bayu WibowoNo ratings yet
- Coa-Stpp-W032022-Chongqing (21.04.22)Document1 pageCoa-Stpp-W032022-Chongqing (21.04.22)Nha TranNo ratings yet
- Product DetailsDocument3 pagesProduct DetailsBiopharma GuruNo ratings yet
- Specification Glycine Ansun 2022Document2 pagesSpecification Glycine Ansun 2022Matt RatcliffeNo ratings yet
- COA Garcinia Cambogia Extract HCA 60Document1 pageCOA Garcinia Cambogia Extract HCA 60Ali Zainal Abidin100% (1)
- CoA HYDROXYLAMINE HYDROCHLORIDE Loba ChemieDocument1 pageCoA HYDROXYLAMINE HYDROCHLORIDE Loba ChemieimamaptNo ratings yet
- HISTIDINEDocument1 pageHISTIDINEAhmedNo ratings yet
- Lampiran 3 Dan 4 Proposal DisertasiDocument2 pagesLampiran 3 Dan 4 Proposal DisertasioktariyanaNo ratings yet
- Ketamine HCL COA With MLT. SUPRIYADocument3 pagesKetamine HCL COA With MLT. SUPRIYARao Fahim NazarNo ratings yet
- Acido Benzoico Food Grade - Lot 20201026 - TianjiaDocument1 pageAcido Benzoico Food Grade - Lot 20201026 - TianjiaArmando GarciaNo ratings yet
- Beauty CreamDocument1 pageBeauty Creammuhammad imran azizNo ratings yet
- SAPP FG - SpecificationDocument1 pageSAPP FG - SpecificationNha TranNo ratings yet
- SampleDocument2 pagesSampleSeara FerminoNo ratings yet
- Certificate of Analysis: Product: Other Name(s) : CAS No.: Code: Mol. Formula: Mol. WeightDocument1 pageCertificate of Analysis: Product: Other Name(s) : CAS No.: Code: Mol. Formula: Mol. WeightAnonymous yr4a85No ratings yet
- Technical Data Sheet Prodcut Name: L-Carnitine SpecificationDocument1 pageTechnical Data Sheet Prodcut Name: L-Carnitine SpecificationKarin ZamudioNo ratings yet
- General Specification: Muira Puama Extract Poeder (Ratio 10:1)Document5 pagesGeneral Specification: Muira Puama Extract Poeder (Ratio 10:1)melimaulani-1No ratings yet
- COA-Peppermint OilDocument1 pageCOA-Peppermint Oillipengw518No ratings yet
- Certificate of Analysis: Hubei Xingfa Chemicals Group Co., LTDDocument1 pageCertificate of Analysis: Hubei Xingfa Chemicals Group Co., LTDSpecialty Chemicals100% (1)
- Amp Pow PDFDocument1 pageAmp Pow PDFTan YoongNo ratings yet
- Stability Data For Amitraz, 98%Document8 pagesStability Data For Amitraz, 98%Ronald NyamurowaNo ratings yet
- Certificate of AnalysisDocument1 pageCertificate of AnalysisDana JuarezNo ratings yet
- PrintDocument2 pagesPrintShorup GhoshNo ratings yet
- Ascorbic Acid-Shandong LuweiDocument1 pageAscorbic Acid-Shandong LuweiSai Kiran PalikaNo ratings yet
- Auro Labs LTD - Metf HCL USPDocument1 pageAuro Labs LTD - Metf HCL USPsuriana limNo ratings yet
- Biomedical Electron Microscopy: Illustrated Methods and InterpretationsFrom EverandBiomedical Electron Microscopy: Illustrated Methods and InterpretationsNo ratings yet
- EMA Statement On NDMA 1Document2 pagesEMA Statement On NDMA 1windli2012No ratings yet
- 反渗透(Ro)多久消毒一次Document1 page反渗透(Ro)多久消毒一次windli2012No ratings yet
- Introduction 简介: Data Migration ChallengesDocument8 pagesIntroduction 简介: Data Migration Challengeswindli2012No ratings yet
- 制药行业需要一个统一的标准清洁残留限度: 25 Mg m2Document9 pages制药行业需要一个统一的标准清洁残留限度: 25 Mg m2windli2012No ratings yet
- 如何确认数据迁移过程的数据完整性问题Document13 pages如何确认数据迁移过程的数据完整性问题windli2012No ratings yet
- 洛施德GMP咨询 - EU 人用药所用辅料的GMP水平确定用正式风险评估指南 PDFDocument8 pages洛施德GMP咨询 - EU 人用药所用辅料的GMP水平确定用正式风险评估指南 PDFwindli2012No ratings yet
- Human Error Deviations How You Can Stop Creating - Most of - Them PDFDocument13 pagesHuman Error Deviations How You Can Stop Creating - Most of - Them PDFwindli2012No ratings yet
- Assets-AR050314 Article050114 Issue Apprais Laser Diffr Part Siz Techn Tcm54-17970Document6 pagesAssets-AR050314 Article050114 Issue Apprais Laser Diffr Part Siz Techn Tcm54-17970windli2012No ratings yet
- Assets-AR050314 Article050114 Issue Apprais Laser Diffr Part Siz Techn Tcm54-17970Document6 pagesAssets-AR050314 Article050114 Issue Apprais Laser Diffr Part Siz Techn Tcm54-17970windli2012No ratings yet
- Assets TN101104AccuracyPrecisionDLS 6 Tcm54 37106Document5 pagesAssets TN101104AccuracyPrecisionDLS 6 Tcm54 37106windli2012No ratings yet
- Assets TN101104AccuracyPrecisionDLS 6 Tcm54 37106Document5 pagesAssets TN101104AccuracyPrecisionDLS 6 Tcm54 37106windli2012No ratings yet
- Assets TN121120ReproducPSmeasMS3000 6 Tcm54 37740Document6 pagesAssets TN121120ReproducPSmeasMS3000 6 Tcm54 37740windli2012No ratings yet
- ECA GMP Meets DevelopmentDocument4 pagesECA GMP Meets Developmentwindli2012No ratings yet
- Assets TN10110421CFRPart11UserGuideSpraytec97 6 Tcm54 36984Document15 pagesAssets TN10110421CFRPart11UserGuideSpraytec97 6 Tcm54 36984windli2012No ratings yet
- Assets TN101104IntensityVolumeNumber 6 Tcm54 36773Document6 pagesAssets TN101104IntensityVolumeNumber 6 Tcm54 36773windli2012No ratings yet
- Assets TN101104AccuracyPrecisionDLS 6 Tcm54 37106Document5 pagesAssets TN101104AccuracyPrecisionDLS 6 Tcm54 37106windli2012No ratings yet
- FAQs For CAPA Webinar FinalDocument2 pagesFAQs For CAPA Webinar Finalwindli2012No ratings yet
- Assets TN10110421CFRPart11UserGuideSpraytec97 6 Tcm54 36984Document15 pagesAssets TN10110421CFRPart11UserGuideSpraytec97 6 Tcm54 36984windli2012No ratings yet
- Corrective ActionDocument1 pageCorrective Actionwindli2012No ratings yet
- Chap 6 GlycosidesDocument66 pagesChap 6 GlycosidesUkash sukarmanNo ratings yet
- Experiment 2 OrgChem - EditedDocument8 pagesExperiment 2 OrgChem - EditedAntonio CharismaNo ratings yet
- Inactive Ingredients A-Z IndexDocument1 pageInactive Ingredients A-Z IndexcryptoNo ratings yet
- Daftar Harga Pt. Prima Medika Bogor Produk GenerikDocument22 pagesDaftar Harga Pt. Prima Medika Bogor Produk GenerikjohanlyNo ratings yet
- Reactive Printing PDFDocument9 pagesReactive Printing PDFshivanshNo ratings yet
- Epdm & FKM Chemical Resistance GuideDocument42 pagesEpdm & FKM Chemical Resistance GuideMauro MascheroniNo ratings yet
- Acid Base Flipped Notes HWDocument5 pagesAcid Base Flipped Notes HWDayanara Davila BermeoNo ratings yet
- Hydrolysis of Methyl Salicylate ExpDocument7 pagesHydrolysis of Methyl Salicylate ExpPradeep100% (1)
- L1 MudlogDocument9 pagesL1 MudlogЕлнур ИкимбаевNo ratings yet
- Resource Management Strategy of Limestone by Evolution of Nil Waste ProcessDocument3 pagesResource Management Strategy of Limestone by Evolution of Nil Waste ProcessravibelavadiNo ratings yet
- Titration Calculations: Tutor: L. Abiram Course: Edexcel AS Level Date: 28.4.2017Document8 pagesTitration Calculations: Tutor: L. Abiram Course: Edexcel AS Level Date: 28.4.2017Abhi RamNo ratings yet
- Antacid AnalysisDocument84 pagesAntacid AnalysisRajeswari Raji0% (1)
- Portland Cement: Wednesday, March 25, 2015Document35 pagesPortland Cement: Wednesday, March 25, 2015EFRA BININo ratings yet
- S-C-6-3 - Predicting The Polarity of A Molecule and KEYDocument2 pagesS-C-6-3 - Predicting The Polarity of A Molecule and KEYAndrea Gamutan100% (1)
- Laporan Prak. Kimor Asam Amino, Protein, Dan LipidDocument19 pagesLaporan Prak. Kimor Asam Amino, Protein, Dan LipidNvtrNo ratings yet
- 2002 Thallium Review 2002 JEMEPDocument34 pages2002 Thallium Review 2002 JEMEPMINANo ratings yet
- Mixed Topic Revision 4Document18 pagesMixed Topic Revision 4YaakkwNo ratings yet
- Mapping Data KFA - 20230119Document2,003 pagesMapping Data KFA - 20230119RSKB Islam CawasNo ratings yet
- Ionic CompoundDocument21 pagesIonic CompoundRhona AngelaNo ratings yet
- What Happens When Hydrochloric Acid & Sodium Thiosulphate ReactDocument6 pagesWhat Happens When Hydrochloric Acid & Sodium Thiosulphate ReactAzel OthmanNo ratings yet
- Ch08-Testbank PDFDocument30 pagesCh08-Testbank PDFAdrienne Chelsea GabayNo ratings yet
- Enfamil® Liquid Human Milk Fortifier High ProteinDocument13 pagesEnfamil® Liquid Human Milk Fortifier High ProteinMarioNo ratings yet
- Мембрана TM810 SpecDocument2 pagesМембрана TM810 SpecAlexanderNo ratings yet
- Harcourt Essen ReactionDocument2 pagesHarcourt Essen ReactionMohammed Saqlain100% (2)
- WINSEM2021-22 CHE1014 TH VL2021220501387 Reference Material I 03-03-2022 Module 5 Purification of Petroleum ProductsDocument65 pagesWINSEM2021-22 CHE1014 TH VL2021220501387 Reference Material I 03-03-2022 Module 5 Purification of Petroleum ProductsJod KillerNo ratings yet
- Form 2 ACID AND ALKALI SHORTS NOTESDocument27 pagesForm 2 ACID AND ALKALI SHORTS NOTESShahrul HisyamNo ratings yet
- Modul KimiaDocument57 pagesModul KimiaAZIE207No ratings yet
- NLC Intern Dec-JanDocument39 pagesNLC Intern Dec-JanSurya SuryaNo ratings yet
- USEPA Aminoantipryne Metoda HACHDocument6 pagesUSEPA Aminoantipryne Metoda HACHdark_knight007No ratings yet