Professional Documents
Culture Documents
COA Panax Ginseng Ext
Uploaded by
Ferdian Iwank Iriyanto0 ratings0% found this document useful (0 votes)
23 views1 pageOriginal Title
COA Panax ginseng ext
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
23 views1 pageCOA Panax Ginseng Ext
Uploaded by
Ferdian Iwank IriyantoCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
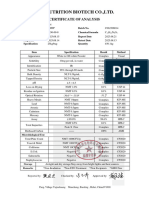
SAMPLE
CERTIFICATE OF ANALYSIS
Product Name Panax Ginseng Extract
Botanical Name Panax ginseng C.A.Mey.
Parts Used Root
Solvent Used Water (50%) & Ethanol (50%)
Grade Food Grade
Solvent Used CRS-A-822828
Manufacturing Date 2018-01-21
Expiry Date 2021-01-20
Irradiation Non-irradiated
TESTS SPECIFICATION RESULTS TEST TEST PROTOCOL
ASSAY
Ginsenosides >5% 5.22 UV
Extract Ratio 5:1 5:1 /
PHYSICAL CONTROL
Appearance Light Yellow fine powder Conforms Visual
Identification Positive Positive HPLC
Taste and Odor Characteristic Conforms Organoleptic
Particle size 100% through 80 mesh Conforms USP
Loss on Drying ≤5.0% 3.30% Eur.Ph.7.0
Bulk Density 30-60 g/100ml 56g/100ml Eur.Ph.
CHEMICAL CONTROL
Heavy Metals ≤10ppm <10ppm ICP-MS
Lead (Pb) ≤2ppm 0.0166ppm ICP-MS
Arsenic (As) ≤2ppm 0.0153ppm ICP-MS
Cadmium (Cd) ≤1ppm 0.0161ppm ICP-MS
Mercury (Hg) ≤0.1ppm 0.01ppm ICP-MS
Solvent Residue Meet standard Conforms GC
MICROBIOLOGICAL CONTROL
Total plate Count ≤10,000cfu/g 15cfu/g USP
Yeast & Mold ≤1000cfu/g 10cfu/g USP
Salmonella Negative Negative USP
E.Coli Negative Negative USP
S. aureus Negative Negative USP
STORAGE
Store in a well-sealed container away from direct sunlight and moisture.
Note: The above information based on the certificate of analysis
received from the manufacturer of this product and not intended
as a substitute for strict quality control analysis by the purchaser Reviewed by : _______________________________
of this product. Tahir Habib (M.Pharm)
You might also like
- Lampiran 3 Dan 4 Proposal DisertasiDocument2 pagesLampiran 3 Dan 4 Proposal DisertasioktariyanaNo ratings yet
- 1, Water Soluble Ginkgo Biloba ExtractDocument1 page1, Water Soluble Ginkgo Biloba ExtractdanilriosNo ratings yet
- Garcinia Cambogia Certificate of AnalysisDocument1 pageGarcinia Cambogia Certificate of AnalysisAli Zainal Abidin100% (1)
- Ginseng leaf ExtractDocument1 pageGinseng leaf Extractyousfinadjah5No ratings yet
- COA of Fenugreek Powder HP-MET-2303001Document1 pageCOA of Fenugreek Powder HP-MET-2303001Saransh singh BarhaiyaNo ratings yet
- Aloe Vera PowderDocument1 pageAloe Vera PowderCahana Rastra CotamaNo ratings yet
- General Specification: Muira Puama Extract Poeder (Ratio 10:1)Document5 pagesGeneral Specification: Muira Puama Extract Poeder (Ratio 10:1)melimaulani-1No ratings yet
- COA of Angelica Sinensis PowderDocument1 pageCOA of Angelica Sinensis PowderJone YingNo ratings yet
- Spec Coleus 10%Document1 pageSpec Coleus 10%marketing splsgroupNo ratings yet
- Coa Grape SeedDocument1 pageCoa Grape SeedVinny Fitria ArdyaniNo ratings yet
- HYA-ACT XS Certificate of AnalysisDocument3 pagesHYA-ACT XS Certificate of AnalysisDIANELANo ratings yet
- EspecifisteviaDocument2 pagesEspecifisteviaCarlos H. Rocha BeltranNo ratings yet
- PDS-oil - Rev 2Document1 pagePDS-oil - Rev 2Toon ju lienNo ratings yet
- COA of Capsicum Annuum PowderDocument1 pageCOA of Capsicum Annuum PowderJone YingNo ratings yet
- GSE - 011 SpecificationDocument1 pageGSE - 011 SpecificationBala NairNo ratings yet
- Ashwagandha Extract Powder 8 % COADocument1 pageAshwagandha Extract Powder 8 % COADeepak VarmaNo ratings yet
- COA of Prune Extract (Xian)Document1 pageCOA of Prune Extract (Xian)iloveit52252No ratings yet
- Green Coffee Extract Certificate of AnalysisDocument1 pageGreen Coffee Extract Certificate of AnalysisEssamNo ratings yet
- Green Coffee Extract Certificate of AnalysisDocument1 pageGreen Coffee Extract Certificate of AnalysisEssamNo ratings yet
- Bioprex Labs: Certificate of AnalysisDocument1 pageBioprex Labs: Certificate of AnalysisThuy PhanNo ratings yet
- Kingherbs Hawthorn Fruit Extract Certificate of Analysis ReportDocument1 pageKingherbs Hawthorn Fruit Extract Certificate of Analysis ReportSANo ratings yet
- AKBA - 10% CoaDocument1 pageAKBA - 10% CoaDeepak VarmaNo ratings yet
- Certificate of Analysis Arctic CLO Strawberry 16oz - 161973Document1 pageCertificate of Analysis Arctic CLO Strawberry 16oz - 161973jayjonbeachNo ratings yet
- Certificate of Analysis Arctic CLO Strawberry 16oz - 161973 PDFDocument1 pageCertificate of Analysis Arctic CLO Strawberry 16oz - 161973 PDFjayjonbeachNo ratings yet
- 3.29 Sucrose Derivatives Eastman SAIB-100: General InformationDocument14 pages3.29 Sucrose Derivatives Eastman SAIB-100: General InformationIcha ChairunNo ratings yet
- PS 005 - AyuFlexDocument1 pagePS 005 - AyuFlexLuis Castro XtrmNo ratings yet
- Certificate of Analysis for Green Coffee Bean ExtractDocument2 pagesCertificate of Analysis for Green Coffee Bean ExtractalexxNo ratings yet
- Polysorb 85 - 70 - 00 RM COADocument2 pagesPolysorb 85 - 70 - 00 RM COAASHOK KUMAR LENKANo ratings yet
- World/Gmp Grade Isopropyl Alcohol, 99%: Certificate of AnalysisDocument3 pagesWorld/Gmp Grade Isopropyl Alcohol, 99%: Certificate of AnalysisChé FeNo ratings yet
- Specification Aloe Vera Gel Freeze Dried PowderDocument1 pageSpecification Aloe Vera Gel Freeze Dried PowderFernando DuqueNo ratings yet
- Specification - DC Fine and MediumDocument3 pagesSpecification - DC Fine and MediumNurhasanahNo ratings yet
- Certificate of Analysis: Xi'An B-ThrivingDocument1 pageCertificate of Analysis: Xi'An B-Thrivingiloveit52252No ratings yet
- Stevia Analysis ReportDocument1 pageStevia Analysis ReportbadrajivNo ratings yet
- Certificate of Analysis: 17α-Hydroxy Progesterone AcetateDocument2 pagesCertificate of Analysis: 17α-Hydroxy Progesterone Acetatewindli2012No ratings yet
- CoA - Rosemary Antioxidant, Odorless (Organic) - 35495 - 332107 - 027.050 - ENDocument2 pagesCoA - Rosemary Antioxidant, Odorless (Organic) - 35495 - 332107 - 027.050 - ENvalentin.sabatetNo ratings yet
- Vitamin A RM COADocument2 pagesVitamin A RM COAASHOK KUMAR LENKANo ratings yet
- Jiaherb Bearberry P.E. - 10% Arbutin (HPLC) - SPECDocument1 pageJiaherb Bearberry P.E. - 10% Arbutin (HPLC) - SPECdanijelamesarNo ratings yet
- CoA FlexTabDocument2 pagesCoA FlexTabM Zinedine Haryanto SIG LabNo ratings yet
- Aspartate Aminotransferase (AST-GOT) - ColorimetricDocument2 pagesAspartate Aminotransferase (AST-GOT) - ColorimetricGuneyden Guneyden0% (1)
- Kingherbs Gymnema Sylvestre Extract Certificate of AnalysisDocument1 pageKingherbs Gymnema Sylvestre Extract Certificate of AnalysisAtiq Ur-RahmanNo ratings yet
- 5-HTP COADocument1 page5-HTP COAwillyvh99No ratings yet
- garlic granulesDocument1 pagegarlic granulesedward120703No ratings yet
- COA-Carnitine HCL-HengtaiDocument1 pageCOA-Carnitine HCL-Hengtaichurch.hrgNo ratings yet
- COA-TYJE-230525-H2-Via Stevia Extract Steviol Glycosides 90%Document1 pageCOA-TYJE-230525-H2-Via Stevia Extract Steviol Glycosides 90%Sophia XieNo ratings yet
- Specifications: CBD Isolate: 150 Bonaberi, Diedo, Douala Littoral ProvinceDocument1 pageSpecifications: CBD Isolate: 150 Bonaberi, Diedo, Douala Littoral ProvinceChé FeNo ratings yet
- FP SpecDocument1 pageFP SpecRatheeshkumar K S NairNo ratings yet
- Especificação Creatina 200 Mesh BaosuiDocument1 pageEspecificação Creatina 200 Mesh BaosuicesargrifoNo ratings yet
- RiceBranOil COA 1584175940Document2 pagesRiceBranOil COA 1584175940Clarissa Hernandez DiazNo ratings yet
- PrintDocument2 pagesPrintShorup GhoshNo ratings yet
- Green Tea ExtractDocument1 pageGreen Tea Extractajitbadboy2No ratings yet
- Certificate of Analysis: HSWT Aspartame Powder Cardboard Box 25 KG E307070 Pharma GradeDocument2 pagesCertificate of Analysis: HSWT Aspartame Powder Cardboard Box 25 KG E307070 Pharma GradezbelmiloudNo ratings yet
- P18020321 #0 Opq Yl - Opq YlDocument2 pagesP18020321 #0 Opq Yl - Opq YlZainab aboodNo ratings yet
- COA For Organic Moringa Powder - Sample ResportDocument1 pageCOA For Organic Moringa Powder - Sample ResportkaramdoNo ratings yet
- Certificate of Analysis:: Tongkat Ali Roots HA PE 100-1Document1 pageCertificate of Analysis:: Tongkat Ali Roots HA PE 100-1jo nemesisNo ratings yet
- Coa 3220141170Document1 pageCoa 3220141170Andres FarfanNo ratings yet
- Cassie Absolute Oil TDSDocument2 pagesCassie Absolute Oil TDSAlbertoNo ratings yet
- Coa of Bcaa 4-1-1Document1 pageCoa of Bcaa 4-1-1dokterasadNo ratings yet
- Certificado de Analisis: - Certificate of Analysis As Received From Our Supplier - Productos Químicos Gonmisol S.A.Document2 pagesCertificado de Analisis: - Certificate of Analysis As Received From Our Supplier - Productos Químicos Gonmisol S.A.pervaz anwerNo ratings yet
- Practical Handbook of Pharmaceutical Chemistry for M.PharmFrom EverandPractical Handbook of Pharmaceutical Chemistry for M.PharmNo ratings yet
- Clinical Data - REVIVEDocument3 pagesClinical Data - REVIVEFerdian Iwank IriyantoNo ratings yet
- Spec Disodium 5-RibonucleotidesDocument1 pageSpec Disodium 5-RibonucleotidesFerdian Iwank IriyantoNo ratings yet
- SLS Nutra - Year Long Pilot Trial White PaperDocument15 pagesSLS Nutra - Year Long Pilot Trial White PaperFerdian Iwank IriyantoNo ratings yet
- Lampiran I Technical Specification 50% Liquid Caustic SodaDocument1 pageLampiran I Technical Specification 50% Liquid Caustic SodaFerdian Iwank IriyantoNo ratings yet
- 17 AsekDocument2 pages17 AsekFerdian Iwank IriyantoNo ratings yet
- Stability Real Time ReportDocument2 pagesStability Real Time ReportFerdian Iwank IriyantoNo ratings yet
- DSM AsekDocument3 pagesDSM AsekFerdian Iwank IriyantoNo ratings yet
- COA Reviv Capsules (Non-GMO, Vegan)Document1 pageCOA Reviv Capsules (Non-GMO, Vegan)Ferdian Iwank IriyantoNo ratings yet
- Stability Accelerated ReportDocument2 pagesStability Accelerated ReportFerdian Iwank IriyantoNo ratings yet
- Proposed ADI ASIKDocument3 pagesProposed ADI ASIKFerdian Iwank IriyantoNo ratings yet
- Lampiran III - CoA NSF-04Document1 pageLampiran III - CoA NSF-04Ferdian Iwank IriyantoNo ratings yet
- (1980) Approved Lists of Bacterial NamesDocument196 pages(1980) Approved Lists of Bacterial NamesLeonardo LopesNo ratings yet
- GS20210322-0029 - Aptp062 - Regulatory Compliance - KR - PBPDocument1 pageGS20210322-0029 - Aptp062 - Regulatory Compliance - KR - PBPFerdian Iwank IriyantoNo ratings yet
- Process Flow AsekDocument1 pageProcess Flow AsekFerdian Iwank IriyantoNo ratings yet
- Stability Study Protocol OrmegaDocument2 pagesStability Study Protocol OrmegaFerdian Iwank IriyantoNo ratings yet
- STABILITY PROTOCOL - Revised (Sep21)Document2 pagesSTABILITY PROTOCOL - Revised (Sep21)Ferdian Iwank IriyantoNo ratings yet
- GS20210322-0027 - Aptp045 - Regulatory Compliance - JP Reg - Food Sanitation LawDocument1 pageGS20210322-0027 - Aptp045 - Regulatory Compliance - JP Reg - Food Sanitation LawFerdian Iwank IriyantoNo ratings yet
- The Ecology Epidemiology Dan Virulence of EnterococcusDocument9 pagesThe Ecology Epidemiology Dan Virulence of EnterococcusFerdian Iwank IriyantoNo ratings yet
- RM Spec PersimmonDocument1 pageRM Spec PersimmonFerdian Iwank IriyantoNo ratings yet
- Raw Material Info: Saccharina sculpera PowderDocument1 pageRaw Material Info: Saccharina sculpera PowderFerdian Iwank IriyantoNo ratings yet
- English Review PAT S2 1920Document3 pagesEnglish Review PAT S2 1920Ferdian Iwank IriyantoNo ratings yet
- RM Spec Lotus RootDocument1 pageRM Spec Lotus RootFerdian Iwank IriyantoNo ratings yet
- Guidelines For The Evaluation of Probiotics in FoodDocument11 pagesGuidelines For The Evaluation of Probiotics in Foodkadec100% (1)
- Stability Protocol - EDocument2 pagesStability Protocol - EFerdian Iwank IriyantoNo ratings yet
- Manufacturing Process - Pea ProteinDocument2 pagesManufacturing Process - Pea ProteinFerdian Iwank IriyantoNo ratings yet
- Math Review WorksheetDocument4 pagesMath Review WorksheetFerdian Iwank IriyantoNo ratings yet
- English Review PAT S2 1920Document3 pagesEnglish Review PAT S2 1920Ferdian Iwank IriyantoNo ratings yet
- Markisa Nutraceutical PDFDocument15 pagesMarkisa Nutraceutical PDFfitriyani syarifahNo ratings yet
- Flavor Descri Class Natural CheeseDocument15 pagesFlavor Descri Class Natural CheeseFerdian Iwank IriyantoNo ratings yet
- Beteq2010 Part 1Document198 pagesBeteq2010 Part 1Zilmar JustiNo ratings yet
- Job DescriptionDocument4 pagesJob Descriptionnafis hasnayenNo ratings yet
- WBOX 0E-1GANGSIRN Spec SheetDocument1 pageWBOX 0E-1GANGSIRN Spec SheetAlarm Grid Home Security and Alarm MonitoringNo ratings yet
- Cisco VoipDocument37 pagesCisco VoipLino Vargas0% (1)
- Beam 3 Design and AnalysisDocument5 pagesBeam 3 Design and AnalysisCelsoRapiNo ratings yet
- Model Question Papers SolutionDocument39 pagesModel Question Papers SolutionVinayaka Gombi100% (2)
- Principles of Synthetic BiologyDocument21 pagesPrinciples of Synthetic BiologyOpale PapaleNo ratings yet
- HW3 Solutions 2017 SpringDocument4 pagesHW3 Solutions 2017 SpringAtaush Sabuj100% (1)
- The Definition of WorkDocument2 pagesThe Definition of WorkCarlton GrantNo ratings yet
- Partial Molar PropertiesDocument6 pagesPartial Molar PropertiesNISHTHA PANDEYNo ratings yet
- Flexi Edge Bts SystemDocument25 pagesFlexi Edge Bts SystemMuty Koma67% (3)
- Steel Cargoes GuidanceDocument64 pagesSteel Cargoes GuidanceAamir SirohiNo ratings yet
- CH 6 SandwichesDocument10 pagesCH 6 SandwichesKrishna ChaudharyNo ratings yet
- Upper Limb OrthosisDocument47 pagesUpper Limb OrthosisPraneethaNo ratings yet
- Operation and Maintenance of Power Plant PDFDocument31 pagesOperation and Maintenance of Power Plant PDFwonderstrikeNo ratings yet
- DirectionalDocument114 pagesDirectional1234jjNo ratings yet
- Resume Ked 2 1Document2 pagesResume Ked 2 1api-273985023No ratings yet
- Chapter OneDocument18 pagesChapter Oneحيدر محمدNo ratings yet
- KUBOTA MU5501 4WD Tractor - T-1037-1562-2016Document9 pagesKUBOTA MU5501 4WD Tractor - T-1037-1562-2016Prashant PatilNo ratings yet
- GDRatingDocument13 pagesGDRatingdgzaquinojcNo ratings yet
- ExxonMobil History, Strategies, and Financial PerformanceDocument50 pagesExxonMobil History, Strategies, and Financial PerformanceJose FrancisNo ratings yet
- Marketing Plan: Walton NextDocument26 pagesMarketing Plan: Walton NextAnthony D SilvaNo ratings yet
- Tacana Project (15687597)Document1 pageTacana Project (15687597)jesusNo ratings yet
- 2022-2023 Enoch Calendar: Northern HemisphereDocument14 pages2022-2023 Enoch Calendar: Northern HemisphereThakuma YuchiiNo ratings yet
- Mercruiser 4.3L Mpi SpecsDocument2 pagesMercruiser 4.3L Mpi Specssalvatore dalessandro100% (1)
- Rubric in Poster Making PDFDocument1 pageRubric in Poster Making PDFSerolf IanNo ratings yet
- CollegeMathText F2016Document204 pagesCollegeMathText F2016PauloMtzNo ratings yet
- SMEDA (Small and Medium Enterprises Development Authority)Document29 pagesSMEDA (Small and Medium Enterprises Development Authority)Salwa buriroNo ratings yet
- Capital Today FINAL PPMDocument77 pagesCapital Today FINAL PPMAshish AgrawalNo ratings yet
- Cycle Counter: C1Cm/C1Cf C1Sm/C1SfDocument2 pagesCycle Counter: C1Cm/C1Cf C1Sm/C1SfJustin GentryNo ratings yet