Professional Documents
Culture Documents
Amp Pow PDF
Uploaded by
Tan Yoong0 ratings0% found this document useful (0 votes)
15 views1 pageThis certificate of analysis is for a 550kg batch of ampicillin trihydrate produced by North China Pharmaceutical Group Semisyntech Co., Ltd. on August 20, 2014. The results of tests for characteristics, identification, assay, water content, appearance of solution, pH, specific optical rotation, sulphated ash, and related substances all comply with British Pharmacopoeia 2012 specifications. N,N-Dimethylaniline is not used in the manufacturing process.
Original Description:
Original Title
AMP-POW(1).pdf
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis certificate of analysis is for a 550kg batch of ampicillin trihydrate produced by North China Pharmaceutical Group Semisyntech Co., Ltd. on August 20, 2014. The results of tests for characteristics, identification, assay, water content, appearance of solution, pH, specific optical rotation, sulphated ash, and related substances all comply with British Pharmacopoeia 2012 specifications. N,N-Dimethylaniline is not used in the manufacturing process.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
15 views1 pageAmp Pow PDF
Uploaded by
Tan YoongThis certificate of analysis is for a 550kg batch of ampicillin trihydrate produced by North China Pharmaceutical Group Semisyntech Co., Ltd. on August 20, 2014. The results of tests for characteristics, identification, assay, water content, appearance of solution, pH, specific optical rotation, sulphated ash, and related substances all comply with British Pharmacopoeia 2012 specifications. N,N-Dimethylaniline is not used in the manufacturing process.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
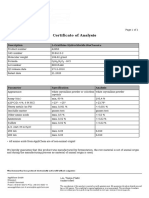
NORTH CHINA PHARMACEUTICAL GROUP SEMISYNTECH CO.,LTD.
CERTIFICATE OF ANALYSIS
AMPICILLIN TRIHYDRATE
BATCH NO.: 1214082009 MANUFACTURING DATE: 20/08/2014
QUANTITY: 550kg REPORT DATE: 23/08/2014
PACKING: 25kg/drum EXPIRATION DATE: 07/2017
ITEMS SPECIFICATIONS RESULTS
Characteristic A white crystalline powder A white crystalline powder
Identification conforms conforms
Assay
96.0% ~ 102.0% 99.1%
(on the anhydrous substance)
Water 12.0% ~ 15.0% 13.5%
Appearance of solution ≤2 1
pH 3.5 ~ 5.5 4.4
Specific optical rotation +280°~ +305° +297°
Sulphated ash ≤0.5% 0.1%
Related substances ≤1.0% 0.2%
CONCLUSION: COMPLIES WITH BP2012
Remark: N,N-Dimethylaniline is
not used in the manufacturing Quality Manager:
process of Ampicillin Trihydrate
Address : No.20 Yangzi Road Shijiazhuang Economic &Technological Development Zone,Hebei,P.R.China
Tel: +86-311-83098234 Fax : +86-311-83098198
Post code: 052165 E-mail: xiantai@sohu.com
You might also like
- AmoxycillinDocument1 pageAmoxycillinEssam Abd AlRahmanNo ratings yet
- L (+) ORNITINA A3450 - 6d015448Document1 pageL (+) ORNITINA A3450 - 6d015448junio16No ratings yet
- Stevia GS90% COADocument1 pageStevia GS90% COAFranck BlauNo ratings yet
- Certificate of Analysis: 17α-Hydroxy Progesterone AcetateDocument2 pagesCertificate of Analysis: 17α-Hydroxy Progesterone Acetatewindli2012No ratings yet
- 二嗪农Document1 page二嗪农缪忠琴No ratings yet
- COA ClonazepamDocument1 pageCOA ClonazepamAli AhmedNo ratings yet
- Celecoxib USP D90 Less Than 10microns Ex Aarti DrugsDocument1 pageCelecoxib USP D90 Less Than 10microns Ex Aarti Drugssuriana limNo ratings yet
- Central Drugs and Pharmaceuticals: Certificate of AnalysisDocument1 pageCentral Drugs and Pharmaceuticals: Certificate of Analysismurugesh bakkiamNo ratings yet
- Pyrazinamida CoaDocument1 pagePyrazinamida Coawillyvh99No ratings yet
- Sorbitol 70% Non Crystallizing Liquid RM COADocument2 pagesSorbitol 70% Non Crystallizing Liquid RM COAASHOK KUMAR LENKANo ratings yet
- COA of Raw MaterialDocument10 pagesCOA of Raw MaterialShafaq ALINo ratings yet
- HISTIDINEDocument1 pageHISTIDINEAhmedNo ratings yet
- Certificate of Analysis: Product: Other Name(s) : CAS No.: Code: Mol. Formula: Mol. WeightDocument1 pageCertificate of Analysis: Product: Other Name(s) : CAS No.: Code: Mol. Formula: Mol. WeightAnonymous yr4a85No ratings yet
- Ketamine HCL COA With MLT. SUPRIYADocument3 pagesKetamine HCL COA With MLT. SUPRIYARao Fahim NazarNo ratings yet
- COA-Carnitine HCL-HengtaiDocument1 pageCOA-Carnitine HCL-Hengtaichurch.hrgNo ratings yet
- AMIKACINA CertificadoDocument1 pageAMIKACINA CertificadoODALIS VELARDE FLORESNo ratings yet
- Turmeric Oil COADocument1 pageTurmeric Oil COAVishal GuptaNo ratings yet
- Certificate of Analysis Triclabendazole: Name of The ProductDocument2 pagesCertificate of Analysis Triclabendazole: Name of The Productbharath kumarNo ratings yet
- Anish Chemicals: 355/2, GIDC, CHITRA, BHAVNAGAR - 364 004 (INDIA) PHONE: + 91-278-2445541, FAX: +91-278-2421009 E-MailDocument1 pageAnish Chemicals: 355/2, GIDC, CHITRA, BHAVNAGAR - 364 004 (INDIA) PHONE: + 91-278-2445541, FAX: +91-278-2421009 E-Mailmonil panchalNo ratings yet
- BAICAO COA of Evening Primrose OilDocument1 pageBAICAO COA of Evening Primrose OilPolyfine Nutra-SciencesNo ratings yet
- Tribulus Terrestris Extract Saponins45%Document2 pagesTribulus Terrestris Extract Saponins45%alimNo ratings yet
- CoA Parasetamol EditDocument2 pagesCoA Parasetamol EditTitin Martini100% (1)
- Hyaluronic Acid Molecular Weight 1.0-1.5 Million DADocument1 pageHyaluronic Acid Molecular Weight 1.0-1.5 Million DAAli SyahabNo ratings yet
- COA-Aluminium Sulphate-Asian PaintsDocument1 pageCOA-Aluminium Sulphate-Asian Paintsdipen royNo ratings yet
- 18mt COA 2210142035Document1 page18mt COA 2210142035nadia ARJDALNo ratings yet
- Product: Camphor White INCI Name: Cinnamomum Camphora Bark Oil CAS No: 92201-50-8 Einecs No: 295-980-1 Countries of Origin: China Batch Code: 102778Document1 pageProduct: Camphor White INCI Name: Cinnamomum Camphora Bark Oil CAS No: 92201-50-8 Einecs No: 295-980-1 Countries of Origin: China Batch Code: 102778ankur mishraNo ratings yet
- COA-Peppermint OilDocument1 pageCOA-Peppermint Oillipengw518No ratings yet
- Coa Polivinilpirrolidona K-90 (PVP K-90) Lote 20221019Document1 pageCoa Polivinilpirrolidona K-90 (PVP K-90) Lote 20221019Ives AlbarracinNo ratings yet
- Caffeine Natural Coffee BeanDocument1 pageCaffeine Natural Coffee BeanMayang TariNo ratings yet
- Certificate of OriginDocument6 pagesCertificate of OriginLUIS STEVEN SANTISTEBAN OBREGONNo ratings yet
- Coa PDFDocument4 pagesCoa PDFsaidNo ratings yet
- Certificate of AnalysisDocument1 pageCertificate of AnalysisDana JuarezNo ratings yet
- Coa 3220141170Document1 pageCoa 3220141170Andres FarfanNo ratings yet
- Ultimo Certificado Etanol Absoluto 4 LITROSDocument1 pageUltimo Certificado Etanol Absoluto 4 LITROSventas emlabNo ratings yet
- Stability Data of Betamethasone XBE USP 20230116Document7 pagesStability Data of Betamethasone XBE USP 20230116Cuauhtemoc Leal VilledaNo ratings yet
- CoA HYDROXYLAMINE HYDROCHLORIDE Loba ChemieDocument1 pageCoA HYDROXYLAMINE HYDROCHLORIDE Loba ChemieimamaptNo ratings yet
- 3-2-S-4 Control of Drug Substances (Assay)Document33 pages3-2-S-4 Control of Drug Substances (Assay)AaminasindhuNo ratings yet
- COA For EPH 3 (10ppm)Document1 pageCOA For EPH 3 (10ppm)Argentus Asesorías QuímicasNo ratings yet
- Certificate of Analysis: Hubei Xingfa Chemicals Group Co., LTDDocument1 pageCertificate of Analysis: Hubei Xingfa Chemicals Group Co., LTDSpecialty Chemicals100% (1)
- COA For EPH 2 25ppmDocument1 pageCOA For EPH 2 25ppmArgentus Asesorías QuímicasNo ratings yet
- Methyl Anthranilate COA PDFDocument1 pageMethyl Anthranilate COA PDFAzkiyah RahmahNo ratings yet
- Auscolor Ss98 Stannous Sulphate.: Technical DataDocument2 pagesAuscolor Ss98 Stannous Sulphate.: Technical DatatayefehNo ratings yet
- Salicylic Acid 69-72-7 COADocument1 pageSalicylic Acid 69-72-7 COASACO QCNo ratings yet
- Certificate of Analysis-VanillinDocument1 pageCertificate of Analysis-VanillinToghrulNo ratings yet
- Coa Grape SeedDocument1 pageCoa Grape SeedVinny Fitria ArdyaniNo ratings yet
- 毒死蜱Document1 page毒死蜱缪忠琴No ratings yet
- Carbomer 940Document11 pagesCarbomer 940Saif KhanNo ratings yet
- COA - Ascorbic Acid, B# 1101070022-2Document1 pageCOA - Ascorbic Acid, B# 1101070022-2Aboudeh FarranNo ratings yet
- Auro Labs LTD - Metf HCL USPDocument1 pageAuro Labs LTD - Metf HCL USPsuriana limNo ratings yet
- Certificate of Analysis: BiotinDocument1 pageCertificate of Analysis: BiotinAchmad LatiefNo ratings yet
- Ficha Tecnica GentamicinaDocument2 pagesFicha Tecnica GentamicinaCarmen AgueroNo ratings yet
- SorbitolDocument1 pageSorbitolZainab aboodNo ratings yet
- Cholecalciferol RM COA 05Document1 pageCholecalciferol RM COA 05ASHOK KUMAR LENKA100% (1)
- Specification Disodium 5'-RibonucleotideDocument1 pageSpecification Disodium 5'-RibonucleotideSlaviša StojanovićNo ratings yet
- COA Elacestrant Dihydrochloride Shandongkehui - 20240120221842Document2 pagesCOA Elacestrant Dihydrochloride Shandongkehui - 20240120221842rashidulhasan789No ratings yet
- 1 Ethyl 3 Methylimidazolium Bis (Trifluoromethylsulfonyl) Imide CoaDocument1 page1 Ethyl 3 Methylimidazolium Bis (Trifluoromethylsulfonyl) Imide CoaAtif JavaidNo ratings yet
- SampleDocument2 pagesSampleSeara FerminoNo ratings yet
- Hangzhou Zhongbao Imp and Exp. Corp., LTDDocument1 pageHangzhou Zhongbao Imp and Exp. Corp., LTDmedicotNo ratings yet
- Xi'an Haoxuan Bio-Tech Co.,Ltd: Certificate of AnylasisDocument1 pageXi'an Haoxuan Bio-Tech Co.,Ltd: Certificate of AnylasisNilsNo ratings yet