Professional Documents
Culture Documents
Pyrazinamida Coa
Uploaded by
willyvh990 ratings0% found this document useful (0 votes)
2 views1 pageCERTIFICADO DE CALIDAD PYRAZINAMIDA
Original Title
PYRAZINAMIDA COA
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCERTIFICADO DE CALIDAD PYRAZINAMIDA
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
2 views1 pagePyrazinamida Coa
Uploaded by
willyvh99CERTIFICADO DE CALIDAD PYRAZINAMIDA
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
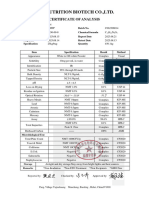
CHANGZHOU YUTIAN BIOLOGICAL AND MEDICAL
TECHNOLOGY CO., LTD.
CERTIFICATE OF ANALYSIS
PRODUCT Pyrazinamide BATCH NO. 20231029
MANU.DATE 2023.10.29 EXP.DATE 2026.10.28
PACKING 25kg/drum QUANTITY 500kg
SUBJECT STANDARD RESULT
Appearance White or almost white crystalline powder Conform
Solubility Meet the requirement Conform
Idenfication A; B; C Conform
Melting point 188~191oC 189.1~190.4oC
Solution Clear and colorless Conform
Impurity B: 0.10% max. N.D.
Related substances Unspecified impurities: 0.05% max. 0.03%
Total impurities: 0.2% max. 0.10%
Heavy metals 10ppm max. <10ppm
Water 0.5% max. 0.03%
Sulfated ash 0.1% max. 0.04%
Assay 99.0~101.0% 99.5%
CONCLUSION: MEETS THE REQUIREMENTS OF BP2014.
Tested by: Xu Tianyu Checked by: Chen Ying Approved by: Xu Ge
You might also like
- Antralina CoaDocument1 pageAntralina Coawillyvh99No ratings yet
- SNC - COA - Toltrazuril 98 - 102%Document1 pageSNC - COA - Toltrazuril 98 - 102%ben grugeNo ratings yet
- Central Drugs and Pharmaceuticals: Certificate of AnalysisDocument1 pageCentral Drugs and Pharmaceuticals: Certificate of Analysismurugesh bakkiamNo ratings yet
- Auro Labs LTD - Metf HCL USPDocument1 pageAuro Labs LTD - Metf HCL USPsuriana limNo ratings yet
- COA of Calcium D Pantothente - RevisedDocument1 pageCOA of Calcium D Pantothente - RevisedJose.SuarezNo ratings yet
- COA AdapaleneDocument1 pageCOA Adapalenewillyvh99No ratings yet
- COA Elacestrant Dihydrochloride Shandongkehui - 20240120221842Document2 pagesCOA Elacestrant Dihydrochloride Shandongkehui - 20240120221842rashidulhasan789No ratings yet
- COA CrosscarmDocument1 pageCOA CrosscarmSouheila MniNo ratings yet
- CoA Sucralose - Supplier Tokped (Shandong Kanbo)Document1 pageCoA Sucralose - Supplier Tokped (Shandong Kanbo)Tantriyani GunadyNo ratings yet
- Ketamine HCL COA With MLT. SUPRIYADocument3 pagesKetamine HCL COA With MLT. SUPRIYARao Fahim NazarNo ratings yet
- FT Vitamina B6 PDFDocument1 pageFT Vitamina B6 PDFrolandoNo ratings yet
- Vitamin B6, Pyridoxine Hydrochloride Ex Tianxin EP7/BP2013/USP36/FCC8Document1 pageVitamin B6, Pyridoxine Hydrochloride Ex Tianxin EP7/BP2013/USP36/FCC8rolandoNo ratings yet
- Coa 3220141170Document1 pageCoa 3220141170Andres FarfanNo ratings yet
- COA - Ascorbic Acid, B# 1101070022-2Document1 pageCOA - Ascorbic Acid, B# 1101070022-2Aboudeh FarranNo ratings yet
- D Panthenol COADocument1 pageD Panthenol COAwillyvh99No ratings yet
- Certificate of AnalysisDocument1 pageCertificate of AnalysisDana JuarezNo ratings yet
- 18mt COA 2210142035Document1 page18mt COA 2210142035nadia ARJDALNo ratings yet
- 1 Coa-DcpDocument1 page1 Coa-DcpjivaorganicNo ratings yet
- COA OF MENTHOL 薄荷脑Document1 pageCOA OF MENTHOL 薄荷脑Gioacchino MondelloNo ratings yet
- Coa-Stpp-W032022-Chongqing (21.04.22)Document1 pageCoa-Stpp-W032022-Chongqing (21.04.22)Nha TranNo ratings yet
- COA of LithoponeDocument1 pageCOA of LithoponechaitanyaNo ratings yet
- Ascorbic Acid-Shandong LuweiDocument1 pageAscorbic Acid-Shandong LuweiSai Kiran PalikaNo ratings yet
- Alginat-Loba ChemicalDocument2 pagesAlginat-Loba ChemicalNur NurkurniaNo ratings yet
- CoA Dequalinium 130Document1 pageCoA Dequalinium 130suriana limNo ratings yet
- CoA FlexTabDocument2 pagesCoA FlexTabM Zinedine Haryanto SIG LabNo ratings yet
- Hangzhou Zhongbao Imp and Exp. Corp., LTDDocument1 pageHangzhou Zhongbao Imp and Exp. Corp., LTDmedicotNo ratings yet
- Xi'an Haoxuan Bio-Tech Co.,Ltd: Certificate of AnylasisDocument1 pageXi'an Haoxuan Bio-Tech Co.,Ltd: Certificate of AnylasisNilsNo ratings yet
- COA For CETYLPYRIDINIUM CHLORIDE MONOHYDRATE USPDocument1 pageCOA For CETYLPYRIDINIUM CHLORIDE MONOHYDRATE USPquimico10012812No ratings yet
- Sodium Carboxymethyl Cellulose (CMC) 9148: Products Data SheetDocument3 pagesSodium Carboxymethyl Cellulose (CMC) 9148: Products Data SheetAldi Ais DarmaNo ratings yet
- BAICAO COA of Evening Primrose OilDocument1 pageBAICAO COA of Evening Primrose OilPolyfine Nutra-SciencesNo ratings yet
- Caffeine Natural Coffee BeanDocument1 pageCaffeine Natural Coffee BeanMayang TariNo ratings yet
- Coa Polivinilpirrolidona K-90 (PVP K-90) Lote 20221019Document1 pageCoa Polivinilpirrolidona K-90 (PVP K-90) Lote 20221019Ives AlbarracinNo ratings yet
- COA of 4 - Hydroxy TEMPODocument1 pageCOA of 4 - Hydroxy TEMPOAhmed WagihNo ratings yet
- Vitamin CDocument2 pagesVitamin CJimmy Bayu WibowoNo ratings yet
- Coa 0893Document1 pageCoa 0893zahid yousufNo ratings yet
- Certificate of Analysis: Calcium GluconateDocument1 pageCertificate of Analysis: Calcium GluconateEfrain Mendoza MartinezNo ratings yet
- CoA HYDROXYLAMINE HYDROCHLORIDE Loba ChemieDocument1 pageCoA HYDROXYLAMINE HYDROCHLORIDE Loba ChemieimamaptNo ratings yet
- COA of Raw MaterialDocument10 pagesCOA of Raw MaterialShafaq ALINo ratings yet
- Certificado de Analisis: - Certificate of Analysis As Received From Our Supplier - Productos Químicos Gonmisol S.A.Document2 pagesCertificado de Analisis: - Certificate of Analysis As Received From Our Supplier - Productos Químicos Gonmisol S.A.pervaz anwerNo ratings yet
- Specification Disodium 5'-RibonucleotideDocument1 pageSpecification Disodium 5'-RibonucleotideSlaviša StojanovićNo ratings yet
- Coa STPP XingfaDocument1 pageCoa STPP XingfaNha TranNo ratings yet
- 5-HTP CoaDocument1 page5-HTP Coawillyvh99No ratings yet
- Beauty CreamDocument1 pageBeauty Creammuhammad imran azizNo ratings yet
- COA of Riboflavin 5 - Sodium Phosphate 23120803Document2 pagesCOA of Riboflavin 5 - Sodium Phosphate 23120803Dwi Satria PutraNo ratings yet
- AKBA - 10% CoaDocument1 pageAKBA - 10% CoaDeepak VarmaNo ratings yet
- COA of Coenzyme Q10Document1 pageCOA of Coenzyme Q10Pan EmmaNo ratings yet
- Amp Pow PDFDocument1 pageAmp Pow PDFTan YoongNo ratings yet
- Technical Specifications (Stim Products)Document3 pagesTechnical Specifications (Stim Products)izzyguyNo ratings yet
- Coa 2010-11 - 23Document1 pageCoa 2010-11 - 23Kuldeep DeshmukhNo ratings yet
- Shaanxi Jiahe Phytochem Co., LTD: Certificate of AnalysisDocument1 pageShaanxi Jiahe Phytochem Co., LTD: Certificate of AnalysisErum Manzoor50% (2)
- Coa. Vit b6Document2 pagesCoa. Vit b6Sakib ChowdhuryNo ratings yet
- Technical Data Sheet: Welsconda Co.,LimitedDocument1 pageTechnical Data Sheet: Welsconda Co.,LimitedAnu ShanthanNo ratings yet
- SampleDocument2 pagesSampleSeara FerminoNo ratings yet
- COA Ascorbic Acid BPDocument1 pageCOA Ascorbic Acid BPVinny MadaanNo ratings yet
- Certificate of Analysis: Product: Other Name(s) : CAS No.: Code: Mol. Formula: Mol. WeightDocument1 pageCertificate of Analysis: Product: Other Name(s) : CAS No.: Code: Mol. Formula: Mol. WeightAnonymous yr4a85No ratings yet
- Certificate of AnalysisDocument1 pageCertificate of AnalysisbluemyNo ratings yet
- Celecoxib USP D90 Less Than 10microns Ex Aarti DrugsDocument1 pageCelecoxib USP D90 Less Than 10microns Ex Aarti Drugssuriana limNo ratings yet
- Spec (BP, EP, USP, E300, FCC) - Vitamin C Ex Ningxia QiyuangDocument2 pagesSpec (BP, EP, USP, E300, FCC) - Vitamin C Ex Ningxia QiyuangPaulo Roberto Baggio MoreiraNo ratings yet
- COA Prilocaine HCL DST 2Document1 pageCOA Prilocaine HCL DST 2charlie ponteNo ratings yet