Professional Documents
Culture Documents
Format Research Paper
Uploaded by
Shivraj PardeshiCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Format Research Paper
Uploaded by
Shivraj PardeshiCopyright:
Available Formats
PLANT DESIGN OF TITANIUM DIOXIDE
MANUFACTURING
Priyanka Kshirsagar Saket Rokde Neha Ravnang Parikshit Sonar
Department of Chemical Engineering Department of Chemical Engineering Department of Chemical Engineering Department of Chemical Engineering
Vishwakarma Institute of Technology Vishwakarma Institute of Technology Vishwakarma Institute of Technology Vishwakarma Institute of Technology
Pune, India Pune, India Pune, India Pune, India
Abstract - Titanium, renowned for its exceptional strength, industry.
corrosion resistance, and low density, has found extensive This project paper aims to provide an in-depth analysis of
applications across diverse industries. However, traditional the titanium dioxide production using the chloride
manufacturing methods have posed several challenges, process. It will explore the fundamental principles, steps
including high costs, limited scalability, and environmental
involved, and the key factors influencing the efficiency
concerns. In response, this project paper introduces a novel
titanium manufacturing process that addresses these and effectiveness of this process. Furthermore, the paper
limitations while enhancing material properties and will evaluate the economic viability and environmental
reducing production timelines. sustainability of the chloride process compared to other
production methods.
The project paper will discuss the experimental setup and The paper will begin by outlining the importance of
methodology used to validate the proposed titanium titanium dioxide as a crucial industrial pigment and its
manufacturing process, including characterization wide range of applications. It will then delve into the
techniques, mechanical testing, and performance evaluation. fundamental principles and chemistry behind the chloride
In conclusion, the novel titanium manufacturing process
process, highlighting the key reactions and
presented in this project paper represents a significant
advancement in material fabrication, overcoming the transformations that occur during the production of
limitations of traditional methods. titanium dioxide. This section will provide a solid
foundation for understanding the subsequent discussions
Keywords – Titanium dioxide, manufacturing process, on process optimization and improvements.
pigments, oxidation, synthesis. Next, the paper will focus on the operational aspects of the
chloride process, including the selection of suitable raw
I. INTRODUCTION materials, the process flow diagram, and the various unit
operations involved. It will examine the key parameters
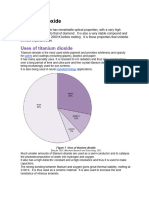
The Titanium dioxide (TiO2) is a versatile and widely that influence the reaction kinetics and product quality,
used white pigment that finds extensive applications in such as temperature, pressure, residence time, and
various industries, including paints, coatings, plastics, feedstock composition. This analysis will provide insights
paper, and cosmetics. It is known for its exceptional into the process optimization strategies and the factors to
opacity, brightness, and UV-protective properties. The consider for achieving high production rates and superior
demand for titanium dioxide has been steadily increasing product quality.
due to its indispensable role in enhancing the performance Furthermore, the paper will evaluate the economic
and aesthetics of numerous consumer and industrial feasibility of the chloride process by examining the capital
products. and operating costs associated with establishing and
The production of titanium dioxide involves different operating a titanium dioxide plant using this method. It
processes, with the chloride process being one of the most will compare these costs with alternative processes to
prominent and efficient methods. The chloride process determine the cost-effectiveness and competitiveness of
the chloride process.
offers several advantages over alternative processes, such Lastly, the paper will address the environmental impact of
as the sulfate process, including higher production rates, the chloride process, considering factors such as energy
improved product quality, and reduced environmental consumption, waste generation, and emissions. It will
impact. These benefits have contributed to the growing discuss the measures and technologies available to
adoption of the chloride process in the titanium dioxide mitigate the environmental effects associated with
titanium dioxide production, such as waste recovery and extraction proved to be highly effective in reducing the
recycling, energy-efficient designs, and emission control
concentration of discoloring impurities in the final
systems. pigment to commercially acceptable levels.
Through this project paper, we aim to provide a A new process for producing commercial quality TiO2
comprehensive understanding of the titanium dioxide pigment features a unique combination of established
production by the chloride process, emphasizing its metallurgical processes, including alkaline roasting of
advantages, challenges, and potential for future titania slag followed by leaching, solvent extraction,
development. By examining the economic and hydrolysis, and calcination . Experimental validation of
environmental aspects, we aim to contribute to the the process chemistry has been demonstrated. Titanium
knowledge base and facilitate informed decision-making recovery was found to be highly dependent on HCl
regarding the choice of titanium dioxide production concentration as insufficient acidity resulted in low
methods. temperature hydrolysis. At a liquids-to-solids ratio of10:1,
leaching with 5 M HCl followed by solvent extraction
with an amine extractant produced a titanium chloride
Problem Statement solution with less than10 ppm Fe. After hydrolysis and
The manufacturing process of Titanium Dioxide (TiO2) is calcination, anatase pigment containing b20 ppm Fe was
currently characterized by high costs and a lack of produced. A forthcoming study compares the estimated
technological advancements, presenting significant energy consumption and carbon emissions of the new
challenges to its production efficiency and widespread process with those of the chloride and sulfate processes.
availability.
II. LITERATURE SURVEY III. METHODOLOGY
Finely dispersed titanium dioxide is widely used in MATERIAL
modern technologies. Proved reserves of titanium ores in Rutile is an oxide mineral composed of titanium dioxide
Russia are expected to meet industrial needs. However, (TiO2), the most common natural form of TiO2. Rarer
the available facilities for the manufacture of titanium polymorphs of TiO2 are known, including anatase,
dioxide from ores are clearly insufficient, which naturally akaogiite, and brookite.
requires new plants to be put into operation. The Rutile has one of the highest refractive indices at visible
technologies used for the production of titanium dioxide wavelengths of any known crystal and also exhibits a
were developed as early as 1940s,when ecological aspects particularly large birefringence and high dispersion.
of the production process were almost not taken into Owing to these properties, it is useful for the manufacture
account. The present survey analyses environmental of certain optical elements, especially polarization optics,
problems related to the titanium dioxide production for longer visible and infrared wavelengths up to about 4.5
according to the sulfate and chloride technologies in micrometers. Natural rutile may contain up to 10% iron
comparison with the fluoride process proposed for and significant amounts of niobium and tantalum.
industrial implementation. Rutile derives its name from the Latin rutilus ('red'), in
Titanium dioxide (TiO2) has been widely used as pigment reference to the deep red color observed in some
in paints, paper and cosmetic products, as well as high- specimens when viewed by transmitted light. Rutile was
tech applications such as solar cells, semiconductors, first described in 1803 by Abraham Gott lob Werner using
biomedical devices and air purification. TiO2 pigment is specimens obtained in Horcajuelo de la Sierra, Madrid
primarily produced by a high temperature chloride (Spain), which is consequently the type locality.
process, which forms CO2 as a reaction byproduct. A
novel hydrometallurgical process for making TiO2 CHLORIDE PROCESS:
pigment without direct CO2 emission is investigated. The The chloride process yields the rutile form of titanium
novel process involves alkaline roasting of titania slag, dioxide .At temperatures between 800 and 1200ºC
with subsequent washing, leaching, solvent extraction, ,chlorine is reacted in a fluidized bed reactor with a
hydrolysis, and calcination stages, resulting in high-purity titanium-containing mineral ,e.g., mineral rutile (which is
anatase or rutile pigments. Experimental validation for not readily attacked by sulfuric acid),under reducing
each of the processing steps is demonstrated. Pigment conditions (presence of coke ) to form anhydrous titanium
whiteness is critically sensitive to trace amounts of (IV) chloride. Purification of the anhydrous tetrachloride
discoloring impurities such as iron. The use of solvent requires separation by fractional condensation.
Conversion of the tetrachloride to titanium dioxide may be PROCESS FLOW DIAGRAM
accomplished by either direct thermal oxidation or
reaction with steam in the vapour phase at temperatures in
the range of 900-1400 ºC. A minor amount of aluminum
chloride is generally added to promote formation of the
rutile form. The titanium dioxide is washed, calcined, and
packaged.
MAIN REACTIONS:
2TiO2+4Cl2+3C 2TiCl4+CO2+2CO(Impure)
TiCl4 + O2 → TiO2 + 2Cl2 (Pure)
PROCESS DESCRIPTION:
In a reactor, titanium dioxide (85 percent pure) and finely
divided coke are added to chlorine gas, where the titanium Figure 1 Process flow Diagram
dioxide is chlorinated at 800 degrees Celsius. The
reactions below produce titanium tetrachloride, as well as
iron and silicon chlorides. VI. RESULTS
The pressure in the reactor is maintained at about 1.5 atm,
and the residence time is roughly 1 hour. The chlorinator's
800°C outlet stream is fed into a cooler and cooled to
320°C using water as a coolant. Condensed iron chloride
settles as a liquid. Unreacted solids settle and are Figure 2 Material balance of chlorinator
separated as well. The condenser receives the vapour
stream from the top .The gas stream from the cooler is
cooled to 137°C, when TiCl4iscondensed.The effluent gas
is subsequently passed through a converter, which
converts carbon monoxide to carbon dioxide .The oxide
burner receives the condensed pure TiCl4 liquid at its
bubble point. Titanium dioxide solid (0.3m particle size)
and chlorine gas are formed when pure TiCl4 liquid is
evaporated and burned with oxygen. At a temperature of
1000°C, the reaction takes place.l4+O2 TiO2+2Cl2
A high-efficiency Cyclone Separator separates the stream
comprising chlorine gas and titanium dioxide solids .The
chlorinator recycles the chlorine gas. The product is
Figure 3 Material balance of cooler
removed, cooled, and surface treated.
Figure 4 Material balance of condenser Figure 7 Material balance of converter
VII. CONCLUSION
The chloride process for titanium dioxide production has
proven to be a highly efficient and cost-effective method.
It offers several advantages over alternative processes,
including higher production rates, improved product
quality, and reduced environmental impact.
Through the chloride process, titanium dioxide is obtained
from natural rutile or ilmenite ores, which are abundant
and widely available. The process involves the
chlorination of the ore, followed by the oxidation of
Figure 5 Material balance of Oxide burner titanium tetrachloride to produce high-purity titanium
dioxide particles.The chloride process for titanium dioxide
production stands as a highly efficient, economically
viable, and environmentally friendly method. Its ability to
achieve high production rates, superior product quality,
and reduced environmental impact makes it an attractive
choice for the manufacturing of titanium dioxide in
various industries. With further advancements and
optimization, the chloride process has the potential to
continue playing a significant role in meeting the growing
global demand.
Figure 6 Material balance of cyclone separator VIII. REFERENCE
[1]. Middlemas, Scott & Fang, Zhigang & Fan, Peng.
(2013). A new method for production of titanium dioxide
pigment. Hydrometallurgy. s 131–132. 107–113.
10.1016/j.hydromet.2012.11.002.
[2]. Gázquez, M.J. & Bolívar, Juan & García-Tenorio,
Rafael & Vaca Galan, Federico. (2014). A Review of the
Production Cycle of Titanium Dioxide Pigment. Materials
Sciences and Applications. 05. 441-458.
10.4236/msa.2014.57048.
[3]. Abdullah Youssef, Abdal-Rhman. (2019). Paints
Industry: Raw materials & unit operations & Equipment
& Manufacturing & Quality tests.
10.13140/RG.2.2.22793.60007.
[4]. A. E. Petersen, M. B. Shirts, and J. P. Allen. (1992).
Production of titanium dioxide pigment from perovskite
concentrates, acid sulfation method; with an appendix on
economic and technical evaluation by J.H. Schwier.
TN23.U43 [TN799.T5] 622 s-dc20 [669'.7322] 89-600336
elP
[5] Balderson, G.F., MacDonald, C.A., 1999. Method for
the production of synthetic rutile. US Patent 5885324.
[6] Barksdale, J., 1966. Titanium: Its Occurrence,
Chemistry, and Technology. Ronald Press Co, New York,
pp. 256–350.
[7] Bauer, G., 1996. 5th edition. Vanadium and Vanadium
Compounds, Ullmann's Encyclopedia of Industrial
Chemistry, vol. A27. Wiley-VCH, Weinheim, Germany,
pp. 367–386.
[8] Borowiec, K., Grau, A.E., Gueguin, M., Turgeon, J.F.,
1998. Method to upgrade titania slag and resulting
product. US Patent 5830420.
[9] Duyvesteyn, W.P.C., Spitler, T.M., Sabacky, B.J.,
Vince, A., Prochazka, J., 2003. Processing aqueous
titanium solutions to titanium dioxide pigment. US Patent
6548039.
[10] European Commission, 2007. Integrated Pollution
Prevention and Control — Best Available Techniques for
the Manufacture of Large Volume Inorganic Chemicals–
solids and Other Industries. European Commission
Report, Seville, Spain, pp. 103–200.
You might also like
- Value-In-Use Model For Chlorination of Titania FeedstocksDocument10 pagesValue-In-Use Model For Chlorination of Titania FeedstocksFelipe TavaresNo ratings yet
- Life Cycle Assessment Comparison of Emerging and Traditional Titanium Dioxide Manufacturing ProcessesDocument11 pagesLife Cycle Assessment Comparison of Emerging and Traditional Titanium Dioxide Manufacturing ProcessesFelipe TavaresNo ratings yet
- Report On Chloro Caustic GroupDocument79 pagesReport On Chloro Caustic Grouppankaj verma100% (2)
- SSRN Id4553127Document5 pagesSSRN Id4553127ghinasaleh97No ratings yet
- Hydrometallurgy: Scott Middlemas, Z. Zak Fang, Peng FanDocument7 pagesHydrometallurgy: Scott Middlemas, Z. Zak Fang, Peng FanmonisalesNo ratings yet
- 5b SRRTTF TiO2 2019 - 02 - 18Document21 pages5b SRRTTF TiO2 2019 - 02 - 18Hamodeh MDNo ratings yet
- 16-04-2020-1587032337-6 - Ijce-2. Ijce - Processing of Rutile Concentrates by FluorinationDocument18 pages16-04-2020-1587032337-6 - Ijce-2. Ijce - Processing of Rutile Concentrates by Fluorinationiaset123No ratings yet
- The Economic Benefits of Chlorine Chemistry in Titanium and Titanium Dioxide in The US and Canada PDFDocument15 pagesThe Economic Benefits of Chlorine Chemistry in Titanium and Titanium Dioxide in The US and Canada PDFAmer AlkalaifhNo ratings yet
- Design and Development of Removing TiO2 Nanoparticles by Coagulation-Flocculation Filtration MethodDocument3 pagesDesign and Development of Removing TiO2 Nanoparticles by Coagulation-Flocculation Filtration MethodAngelica Marie BejerNo ratings yet
- MDMW Iron43Document4 pagesMDMW Iron43miningnovaNo ratings yet
- MJC - UNIKOM - 05 - Hanif Nur Purnamasari (Final Manuscript) - RevDocument11 pagesMJC - UNIKOM - 05 - Hanif Nur Purnamasari (Final Manuscript) - RevMUHAMAD RIDWANNo ratings yet
- Photocatalytic Degradation of H S in The Gas-Phase Using A Continuous Flow Reactor Coated With Tio - Based Acrylic PaintDocument37 pagesPhotocatalytic Degradation of H S in The Gas-Phase Using A Continuous Flow Reactor Coated With Tio - Based Acrylic PaintSyeda Ammara AnwarNo ratings yet
- Applications of Titanium Oxide Nano CoatingDocument7 pagesApplications of Titanium Oxide Nano CoatingDharmik77 DavraNo ratings yet
- 1 s2.0 S2214785323042013 MainDocument6 pages1 s2.0 S2214785323042013 Main17pgme001No ratings yet
- Production of Titanium DioxideDocument16 pagesProduction of Titanium Dioxidehaisamdo100% (1)
- Treatment of Petroleum Industry Wastewater Using Tio2/Uv Photocatalytic ProcessDocument5 pagesTreatment of Petroleum Industry Wastewater Using Tio2/Uv Photocatalytic ProcessBuxNo ratings yet
- Evaluation of Titanium in Hydrochloric Acid SolutiDocument5 pagesEvaluation of Titanium in Hydrochloric Acid SolutiDalila SilveiraNo ratings yet
- Innovation in Metallurgical Waste ManagementDocument3 pagesInnovation in Metallurgical Waste ManagementChu ThuNo ratings yet
- Deia ReviewDocument8 pagesDeia ReviewNuRiAYueNo ratings yet
- A4 PJM ReportDocument14 pagesA4 PJM ReportDharampreet SinghNo ratings yet
- Emerging TitaniumDocument59 pagesEmerging TitaniumThiru MuruganNo ratings yet
- 10.1016 J.jallCOM.2005.10.048 Extraction of Titanium From Different Titania Precursors by The FFC Cambridge ProcessDocument9 pages10.1016 J.jallCOM.2005.10.048 Extraction of Titanium From Different Titania Precursors by The FFC Cambridge ProcessHooman BaghbanNo ratings yet
- UsapeinTongcumpou40881 114657 1 PBDocument9 pagesUsapeinTongcumpou40881 114657 1 PBAbdulrahman JradiNo ratings yet
- GRP BAT Guidelines Steel SectorDocument68 pagesGRP BAT Guidelines Steel SectorSai PrashantNo ratings yet
- Study On Photocatalytic Cement As Solution For Pollution ControlDocument5 pagesStudy On Photocatalytic Cement As Solution For Pollution ControlRAHUL GUNDEBOMMUNo ratings yet
- 1 s2.0 S2666790821001294 Main1Document8 pages1 s2.0 S2666790821001294 Main1AlEm ShEzAr salazarNo ratings yet
- RESUME Chemical Engineering MagazineDocument12 pagesRESUME Chemical Engineering Magazinejihan safNo ratings yet
- Eibc 2335Document39 pagesEibc 2335jhenyNo ratings yet
- Nano Concrete AbstractDocument5 pagesNano Concrete AbstractNArendra REddy100% (2)
- Synthesis of NiO Nanoparticles From Industrial WasteDocument45 pagesSynthesis of NiO Nanoparticles From Industrial WasteGunnar AgarwalNo ratings yet
- Document PDFDocument2 pagesDocument PDFOki Andri Oktaviana0% (1)
- Advanced Powder TechnologyDocument11 pagesAdvanced Powder TechnologyDeepakrao Bornare PatilNo ratings yet
- Document H2o2 Finish New PDFDocument20 pagesDocument H2o2 Finish New PDFdinurjNo ratings yet
- Kang 2016Document8 pagesKang 2016mylover huNo ratings yet
- Submitted To Fulfillment The Bachelor's Degree in Chemical EngineeringDocument19 pagesSubmitted To Fulfillment The Bachelor's Degree in Chemical EngineeringDyala AshrafNo ratings yet
- 27 KTXTDocument14 pages27 KTXTHoàng NhưNo ratings yet
- Applied Catalysis B - Environmental 156-157 (2014) 416-427Document12 pagesApplied Catalysis B - Environmental 156-157 (2014) 416-427toyito20No ratings yet
- Titaniun Dioxide Basic InformationDocument4 pagesTitaniun Dioxide Basic Informationalexandria.padilla11No ratings yet
- Modelling of Solid Recovered Fuel (SRF) Properties Based On Material Composition - Chloride QualityDocument13 pagesModelling of Solid Recovered Fuel (SRF) Properties Based On Material Composition - Chloride QualitySlovenaccNo ratings yet
- PB ExtraccionDocument10 pagesPB ExtraccionMartin ZainaNo ratings yet
- Ipc2022-86892 Pig Sweep Nanoparticles Transform PipelineDocument5 pagesIpc2022-86892 Pig Sweep Nanoparticles Transform PipelineOswaldo MontenegroNo ratings yet
- Ionic Liquid-Assisted Refinery Processes - A Review and Industrial PerspectiveDocument23 pagesIonic Liquid-Assisted Refinery Processes - A Review and Industrial Perspectivemohsen miandehiNo ratings yet
- Cancer CellsDocument57 pagesCancer Cellsrentoclean.indonesiaNo ratings yet
- 3 China VCM PresentationDocument26 pages3 China VCM PresentationfatjonNo ratings yet
- 1 s2.0 S12260adsadas86X13003092 MainDocument9 pages1 s2.0 S12260adsadas86X13003092 MainFabiano Luiz NavesNo ratings yet
- Hydrometallurgy,: 1 - 4 1 Science Publishers B.V., Amsterdam - Printed in The NetherlandsDocument4 pagesHydrometallurgy,: 1 - 4 1 Science Publishers B.V., Amsterdam - Printed in The NetherlandsDaniel Alejandro Jara PaineanNo ratings yet
- Benelite Process For Upgradation of IllemniteDocument6 pagesBenelite Process For Upgradation of Illemnitemadangk100% (1)
- 1355 - 20 Years of Advances in Technology For Chemical CleaningDocument63 pages1355 - 20 Years of Advances in Technology For Chemical CleaningPaulo LiraNo ratings yet
- Iluka-Zircon and OtherDocument13 pagesIluka-Zircon and OtherArief MahdiNo ratings yet
- Irjet V7i10260Document8 pagesIrjet V7i10260Đoàn NgọcNo ratings yet
- Modification and Performances of Tio Photocatalyst Towards Degradation of Paraquat DichlorideDocument10 pagesModification and Performances of Tio Photocatalyst Towards Degradation of Paraquat DichloridenezarahayuNo ratings yet
- Process Development For The Removal of Iron From Nitrided IlmeniteDocument167 pagesProcess Development For The Removal of Iron From Nitrided IlmeniteDeta pratiwiNo ratings yet
- Nanomaterials 10 01854 v3Document47 pagesNanomaterials 10 01854 v3AntonioNo ratings yet
- Application of Titanium Dioxide in Cement and Concrete TechnologyDocument8 pagesApplication of Titanium Dioxide in Cement and Concrete Technologysampath wickramasenaNo ratings yet
- 2018 WER Volume 90 Issue 5 MayDocument14 pages2018 WER Volume 90 Issue 5 Maymohammed mohammedNo ratings yet
- Current Research Trends of Microbiological Leaching For Metal Recovery From Industrial WastesDocument9 pagesCurrent Research Trends of Microbiological Leaching For Metal Recovery From Industrial WastesGabriella WidjajaNo ratings yet
- Wipo Pub 1077 23 en Patent Landscape Report IlmeniteDocument56 pagesWipo Pub 1077 23 en Patent Landscape Report Ilmenitekaushikneha24No ratings yet
- (TTPL) - TTPL Is A Public Limited Company Under The State Public Sector WithDocument56 pages(TTPL) - TTPL Is A Public Limited Company Under The State Public Sector WithShahen MohamedNo ratings yet
- Almaun F1C113044Document25 pagesAlmaun F1C113044Almaun MaunNo ratings yet
- Chem Principles 7e ISM Focus 04 Even FINALDocument62 pagesChem Principles 7e ISM Focus 04 Even FINALSelma MeloNo ratings yet
- Skema Pppa Kimia k2 2014 (Set 1)Document10 pagesSkema Pppa Kimia k2 2014 (Set 1)Siva Guru0% (1)
- Single Phase Smart MeterDocument1 pageSingle Phase Smart MeterBehailu FelekeNo ratings yet
- Tropical Architecture: Green ArchitrendsDocument2 pagesTropical Architecture: Green ArchitrendsBRIAN DIQUIATCONo ratings yet
- ECU AnnalysisDocument6 pagesECU AnnalysisAchmad AminNo ratings yet
- Compressor ManualDocument68 pagesCompressor ManualyosafatedenNo ratings yet
- GTO RectifierDocument13 pagesGTO RectifierBijay PoudelNo ratings yet
- SY Mech Thermo Chapter 1 ConceptsDocument57 pagesSY Mech Thermo Chapter 1 Conceptsananyag396No ratings yet
- How To Create An Effective Social Marketing Campaign: Richard EarleDocument46 pagesHow To Create An Effective Social Marketing Campaign: Richard Earlejana578No ratings yet
- ISO Steam TrapDocument2 pagesISO Steam TrapLuis Felipe ZuñigaNo ratings yet
- Cook Book For Building A Food DehydratorDocument20 pagesCook Book For Building A Food DehydratorLeticia Barreto100% (1)
- DCR 2004 and 2026Document103 pagesDCR 2004 and 2026karthikaNo ratings yet
- Penstock Quality ManagementDocument7 pagesPenstock Quality ManagementaudiihussainNo ratings yet
- ChE 122 LE1 NotesDocument4 pagesChE 122 LE1 Notesgoogley71No ratings yet
- Btech Civil 7to8semDocument62 pagesBtech Civil 7to8semfanishNo ratings yet
- HFGHTFDocument19 pagesHFGHTFWeldayNo ratings yet
- Department of Chemistry Maubin UniversityDocument20 pagesDepartment of Chemistry Maubin Universityေအာင္ ေက်ာ္ စြာNo ratings yet
- Project 4 - Biogas PlantDocument5 pagesProject 4 - Biogas PlantUjjval NagotaNo ratings yet
- EPDM Weatherstrip PerformanceDocument17 pagesEPDM Weatherstrip PerformanceLuciano100% (1)
- MQ137 (Ver1.4) - ManualDocument6 pagesMQ137 (Ver1.4) - ManualAleX KasperNo ratings yet
- Christensen CS3001 Truck Mounted Core DrillDocument2 pagesChristensen CS3001 Truck Mounted Core DrillJulio Jesus Quijano VargasNo ratings yet
- LADWP Power Rate Proposal Cost Reduction ReportDocument143 pagesLADWP Power Rate Proposal Cost Reduction ReportBill RosendahlNo ratings yet
- Compressor Technical Data: NJ9232GK 220-240 V 50 HZ 943NA01 A - Application / Limit Working ConditionsDocument3 pagesCompressor Technical Data: NJ9232GK 220-240 V 50 HZ 943NA01 A - Application / Limit Working ConditionsGeri SulanjakuNo ratings yet
- 4th Periodic Test Grade 9 ScienceDocument4 pages4th Periodic Test Grade 9 ScienceMark Ryan J Bacus75% (4)
- T10 - 2011 - Tender DocumentDocument38 pagesT10 - 2011 - Tender DocumentblindjaxxNo ratings yet
- External FlowDocument21 pagesExternal FlowCristopher Da SilvaNo ratings yet
- FluidMechanics IOE Old Questions-2Document11 pagesFluidMechanics IOE Old Questions-2Abishek AdhikariNo ratings yet
- BE MechDocument218 pagesBE MechBala RaviNo ratings yet
- JCB Lubricants - 2021Document19 pagesJCB Lubricants - 2021Сергій БоженкоNo ratings yet
- 2900299-03 PLC RPT 24DC 21Document4 pages2900299-03 PLC RPT 24DC 21Bsd FareedNo ratings yet
- The Fabric of Civilization: How Textiles Made the WorldFrom EverandThe Fabric of Civilization: How Textiles Made the WorldRating: 4.5 out of 5 stars4.5/5 (57)
- The Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaFrom EverandThe Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaNo ratings yet
- Transformed: Moving to the Product Operating ModelFrom EverandTransformed: Moving to the Product Operating ModelRating: 4 out of 5 stars4/5 (1)
- The End of Craving: Recovering the Lost Wisdom of Eating WellFrom EverandThe End of Craving: Recovering the Lost Wisdom of Eating WellRating: 4.5 out of 5 stars4.5/5 (80)
- Faster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestFrom EverandFaster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestRating: 4 out of 5 stars4/5 (28)
- ChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindFrom EverandChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindNo ratings yet
- Sully: The Untold Story Behind the Miracle on the HudsonFrom EverandSully: The Untold Story Behind the Miracle on the HudsonRating: 4 out of 5 stars4/5 (103)
- Hero Found: The Greatest POW Escape of the Vietnam WarFrom EverandHero Found: The Greatest POW Escape of the Vietnam WarRating: 4 out of 5 stars4/5 (19)
- Mini Farming: Self-Sufficiency on 1/4 AcreFrom EverandMini Farming: Self-Sufficiency on 1/4 AcreRating: 4 out of 5 stars4/5 (76)
- A Place of My Own: The Architecture of DaydreamsFrom EverandA Place of My Own: The Architecture of DaydreamsRating: 4 out of 5 stars4/5 (242)
- The Intel Trinity: How Robert Noyce, Gordon Moore, and Andy Grove Built the World's Most Important CompanyFrom EverandThe Intel Trinity: How Robert Noyce, Gordon Moore, and Andy Grove Built the World's Most Important CompanyNo ratings yet
- The Technology Trap: Capital, Labor, and Power in the Age of AutomationFrom EverandThe Technology Trap: Capital, Labor, and Power in the Age of AutomationRating: 4.5 out of 5 stars4.5/5 (46)
- The Future of Geography: How the Competition in Space Will Change Our WorldFrom EverandThe Future of Geography: How the Competition in Space Will Change Our WorldRating: 4.5 out of 5 stars4.5/5 (4)
- Reality+: Virtual Worlds and the Problems of PhilosophyFrom EverandReality+: Virtual Worlds and the Problems of PhilosophyRating: 4 out of 5 stars4/5 (24)
- The Weather Machine: A Journey Inside the ForecastFrom EverandThe Weather Machine: A Journey Inside the ForecastRating: 3.5 out of 5 stars3.5/5 (31)
- Packing for Mars: The Curious Science of Life in the VoidFrom EverandPacking for Mars: The Curious Science of Life in the VoidRating: 4 out of 5 stars4/5 (1395)
- Pale Blue Dot: A Vision of the Human Future in SpaceFrom EverandPale Blue Dot: A Vision of the Human Future in SpaceRating: 4.5 out of 5 stars4.5/5 (588)
- Designing Data-Intensive Applications: The Big Ideas Behind Reliable, Scalable, and Maintainable SystemsFrom EverandDesigning Data-Intensive Applications: The Big Ideas Behind Reliable, Scalable, and Maintainable SystemsRating: 5 out of 5 stars5/5 (6)
- Lost in a Good Game: Why we play video games and what they can do for usFrom EverandLost in a Good Game: Why we play video games and what they can do for usRating: 4.5 out of 5 stars4.5/5 (31)
- The Path Between the Seas: The Creation of the Panama Canal, 1870-1914From EverandThe Path Between the Seas: The Creation of the Panama Canal, 1870-1914Rating: 4.5 out of 5 stars4.5/5 (124)
- How to Estimate with RSMeans Data: Basic Skills for Building ConstructionFrom EverandHow to Estimate with RSMeans Data: Basic Skills for Building ConstructionRating: 4.5 out of 5 stars4.5/5 (2)