Professional Documents
Culture Documents
Radioactivity and Nuclear Reactions
Uploaded by
dulalsushant30 ratings0% found this document useful (0 votes)
4 views2 pagesOriginal Title
Radioactivity and Nuclear reactions(1)
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
4 views2 pagesRadioactivity and Nuclear Reactions
Uploaded by
dulalsushant3Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
Conceptual Questions 8.
Iodine -131 [53I131] is a radioactive isotope with a decay constant of
1. a. Compare β-particles and γ-rays in terms of their mass, charge, ionizing 9.9 x 10-7 s-1.
power and penetration power. [2] a. State what is meant by radioactivity and decay constant. [2]
b. Define decay constant. [1] b. Obtain a relation between half-life and decay constant. [3]
c. The isotope carbon-14 has half-life 5700 years. If the sample contains c. Some water becomes contaminated with iodine-131. The activity of the
1022 carbon-14 nuclei, find its activity. [2]
iodine – 131 in 1.0kg of water is 560 Bq. Determine the number of
2. Radioactivity is the spontaneously occurring phenomenon in nature.
a. What is radioactivity? [1] iodine-131 atoms in 1.0kg of water. [1]
b. Obtain N = 𝑁𝑜 𝑒 −𝜆𝑡 in radioactive decay law. [3] d. Regulations require that the activity of iodine -131 in 1.0kg of water is
c. Describe the significance of decay curve showing the longest life time to be less than 170 Bq. Calculate the time, in days, for the activity of
of radio isotopes. [1] the contaminated water in (i) to be reduced to 170 Bq. [2]
d. The half-life of radium is 1620 years. After how many years 25% of a 9. a. Define the terms half-life and mean life of a radioactive substance.
radium block remains undecayed? [3] What is relationship between them? [2]
3. a. State radioactivity decay laws. [2] b. The graph shows the percentage of a
b. A sample of radioactive isotopes contains 50% of its original number in radioactive isotope that remains as
2 years. Then, time passes. A student measures the
i. What is its half-life? [1] initial rate of emission of this isotope
ii. If there are 106 such nuclei remaining after 8 years, how many as 40000 emissions per second.
numbers are there in the beginning? [2] i. What is the value of Half-life of
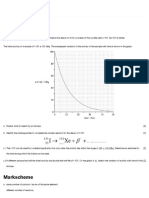
4. Figure shows the decay of radioactive given radio-active substance? [1]
Caesium, 134 55Cs. ii. Determine the average life time of
a. Use the graph to determine the half- given radioactivity material. [1]
life of nuclide in years. [2] How long does it take for the rate of
b. Find the decay constant of the emission to fall to 5000 emissions per second? [1]
radioactive decay of the nuclide. [3] 10. The following graph shows the decay of
c. What would be the remaining radioactive Caesium 55Cs134 . Study the graph
activity after 12 years? [2] and answer the questions.
d. State one application of the a. Heavy, unstable nuclei usually decay by
radioactive decay. [1] emitting an α or β particle. Why don’t they
5. The graph alongside shows the usually emit a single proton or neutron? [2]
count rate recorded when a b. Find the half-life period of Cs. [1]
sample of isotope indium-116 c. Write an equation which represents the
decays. given graph. [1]
a. What is radioactive decay? [1] d. What is initial number of atoms present in
b. Use the graph to find half of the sample? [2]
the isotope. [2] iii. Find the number of atoms present in the sample after 4 years have
c. Calculate decay constant. [3] elapsed. [2]
d. What percent of nuclide has 11. A student measured the activity of a sample of radioactive rock. Her results
been decayed at time t = 32 are presented in the graph.
seconds? [2] a. Explain why the data are scattered. [1]
6. Different isotopes undergo decay to give Before decay After decay b. Determine the half-life of this sample. [2]
isotopes of another element as shown in 14
6𝐶
14
7𝑁 c. How will the shape of this curve will change if she repeats the
the table. 227
90 𝑇ℎ 223
88𝑅𝑎
experiment with a sample with a larger decay constant. Give reason to
a. Determine the types of decay shown your answer. [2]
in the above table. [2] e.
b. State any two properties of γ-rays. [2] 12. Radioactivity is a random process and occurs by chance.
c. How are γ-rays different from α and β particles? [2] a. Comment on the statement “A nucleus contains no electrons and yet
d. How are β particles emitted from nucleus, though electrons are not in can eject them.” [2]
the nucleus? [2] b. Discuss α, β and γ decay. [3]
7. a. A nucleus contains no electrons, yet it ejects them. Explain. [2] 1 𝑡ℎ
A radioactive source has decayed to of its initial activity after 50 days.
b. A radioactive source has decayed to one tenth of one percent of its 28
initial activity in 100 days. What is its half-life period? [3] What is its half-life? [3]
13. A sample of radioactive substance may decay by the emission of either
alpha, beta or gamma-radiation.
a. State the type of radiation which contains (i)leptons (ii)quarks
(iii)particle that remains un deflected in field [3]
c. Which particle alpha, beta or gamma has largest ratio of charge to
mass? [2]
14. In the diagram radioactive source is placed in a cavity.
a. Label A, B and C in the given figure. Which element
is preferred to use as the cavity? [2]
b. The radioactive source decayed to one tenth of one
percent of its initial activity in 100 days. Calculate its
half-life period [3]
15. a. Two radioactive samples A and B have equal number of atoms initially.
Their half-life are 3 hours and 9 hours respectively. Compare their rate
of disintegration after 18 hours from start. [3]
b. What percentage of atoms are decayed after five half-life periods? Show
with calculation. [2]
c. In a given sample, two radio isotopes A and B are initially present in
the ratio 1:4. The half-life of A and B are 100 years and 50 years
respectively. Find the time after which the amounts of A and B become
equal. [3]
Give reasons/ answers
1. What do you mean by radiation hazard?
2. What is Gieger muller tube? Describe its working.
3. Explain, why natural radioactive nuclei are nuclei of high mass numbers?
4. All radioactive series terminate at lead as their final product. Why?
5. A radioactive nucleus has a half-life of one year. Will it be completely decayed
at the end of two year?
6. What do you mean by curie?
7. Can a single nucleus emit α-particle, β-particle and γ-ray simultaneously?
Explain.
8. What is meant by carbon-dating? How can we use this method to estimate
age of dead plant?
You might also like
- (Total 1 Mark) : IB Questionbank Physics 1Document4 pages(Total 1 Mark) : IB Questionbank Physics 1Uncharted FireNo ratings yet
- SL Paper 2Document61 pagesSL Paper 2ANA YENo ratings yet
- Nuclear Chem 2010Document7 pagesNuclear Chem 2010neil-lakdawala-8738No ratings yet
- Topic 12.2 FormativeDocument10 pagesTopic 12.2 FormativeAhmad OmarNo ratings yet
- 12 Physics Atoms and Neclei Test 02 PDFDocument1 page12 Physics Atoms and Neclei Test 02 PDFKrishan LohanNo ratings yet
- FY - Nuclear MCQDocument10 pagesFY - Nuclear MCQNeelam KapoorNo ratings yet
- University of Ghana: Answer Question 1 and Two Other QuestionsDocument2 pagesUniversity of Ghana: Answer Question 1 and Two Other Questionsanthony kingsleyNo ratings yet
- CHAPTERWISETEST - D09 Dec 2022Document4 pagesCHAPTERWISETEST - D09 Dec 2022Atharva SisodiyaNo ratings yet
- Particles and Waves Summary Notes 1Document4 pagesParticles and Waves Summary Notes 1Vanessa PassarelloNo ratings yet
- University of Ghana: Answer Question 1 and Two Other QuestionsDocument2 pagesUniversity of Ghana: Answer Question 1 and Two Other Questionsanthony kingsleyNo ratings yet
- Atoms: Half Life Questions and AnswersDocument6 pagesAtoms: Half Life Questions and AnswersBubuNo ratings yet
- Test1 MakeupDocument6 pagesTest1 MakeupananNo ratings yet
- Aakash Cbse Term-Ii - Amtp (Class Xii) - 2021-22Document132 pagesAakash Cbse Term-Ii - Amtp (Class Xii) - 2021-22AadithS mtpprep0% (1)
- Physics Chemistry Biology Mathematics: Aakash Model Test Papers (AMTP)Document64 pagesPhysics Chemistry Biology Mathematics: Aakash Model Test Papers (AMTP)Anjali KunduNo ratings yet
- 7 SL-paper3Document15 pages7 SL-paper3Onur YavuzcetinNo ratings yet
- Phy 441 Exam 2021 DistanceDocument4 pagesPhy 441 Exam 2021 Distanceandrew silungweNo ratings yet
- Exercise 1 - GR 10 - Physics (Sec 5 Nuclear Physics)Document10 pagesExercise 1 - GR 10 - Physics (Sec 5 Nuclear Physics)oktavianusjordanNo ratings yet
- Worksheet 17Document2 pagesWorksheet 17JunLi CaiNo ratings yet
- Page of Paper Trick-4-ScienceDocument2 pagesPage of Paper Trick-4-ScienceKirti SharmaNo ratings yet
- Science Department: Topic 7.1 - Discrete Energy & Radioactivity Topic 7.2 - Nuclear Reactions 12 Grade (Physics SL)Document8 pagesScience Department: Topic 7.1 - Discrete Energy & Radioactivity Topic 7.2 - Nuclear Reactions 12 Grade (Physics SL)ananNo ratings yet
- Radioactive Decay QPDocument10 pagesRadioactive Decay QPJohnNo ratings yet
- 50Q - NucleiDocument8 pages50Q - NucleiAkash SinghNo ratings yet
- AIEEE - Atomic Nucleus - 2Document3 pagesAIEEE - Atomic Nucleus - 2Amit KashyapNo ratings yet
- HL Paper2Document36 pagesHL Paper2Sharon ChanNo ratings yet
- Ka SH AnDocument10 pagesKa SH Ansarav dhanuNo ratings yet
- 11 Atoms and RadioactivityDocument5 pages11 Atoms and RadioactivityGajendraNo ratings yet
- Physical Test - Q - 05.07.2021Document3 pagesPhysical Test - Q - 05.07.2021joydeep17590No ratings yet
- X Sem - CHEMISTRY.Quantum, Nuclear & Radiation Chem - Old CBCS - MSC Ed May 2019Document3 pagesX Sem - CHEMISTRY.Quantum, Nuclear & Radiation Chem - Old CBCS - MSC Ed May 2019Raghavendra BNo ratings yet
- HL Paper 2: Full Electron Configuration of The Ruthenium (II) IonDocument20 pagesHL Paper 2: Full Electron Configuration of The Ruthenium (II) IonfuduNo ratings yet
- Science Grade 9 Sample Paper1Document15 pagesScience Grade 9 Sample Paper1khannavivaan72No ratings yet
- Chapter 10: RadioactivityDocument6 pagesChapter 10: RadioactivitychorianNo ratings yet
- Sample Question Paper Class 9 2023 - 24Document7 pagesSample Question Paper Class 9 2023 - 24navdeepkhokhar061100% (1)
- FY Radio MCQDocument3 pagesFY Radio MCQNeelam KapoorNo ratings yet
- Class Note P2 CH 6,8,9 (EV)Document6 pagesClass Note P2 CH 6,8,9 (EV)Tanzia RahmanNo ratings yet
- Goldengate Int'L College: First Terminal Examination-2080Document2 pagesGoldengate Int'L College: First Terminal Examination-2080sachin shahNo ratings yet
- AIEEE - Atomic Nucleus - 1Document3 pagesAIEEE - Atomic Nucleus - 1Amit KashyapNo ratings yet
- CH - 12 Atom PYQDocument1 pageCH - 12 Atom PYQKrishna ManiNo ratings yet
- ICSE Class 8 Physics Sample Paper 1Document6 pagesICSE Class 8 Physics Sample Paper 1IoNo ratings yet
- Algebra UttamDocument25 pagesAlgebra Uttamjessinbekkam1210No ratings yet
- Science Stage 9 01 8RP AFP tcm143-639991Document17 pagesScience Stage 9 01 8RP AFP tcm143-639991mal0% (1)
- University of Ghana: Answer Question 1 and Two Other QuestionsDocument2 pagesUniversity of Ghana: Answer Question 1 and Two Other Questionsanthony kingsleyNo ratings yet
- PH110 2010 08Document3 pagesPH110 2010 08lyon juniorNo ratings yet
- Cbjescss 10Document9 pagesCbjescss 10Upendra Sinh 11No ratings yet
- Sample Paper - IX Section A - PhysicsDocument3 pagesSample Paper - IX Section A - Physicsvishal_bokaroNo ratings yet
- Science Department: Topic 7.1 - Discrete Energy & Radioactivity Topic 7.2 - Nuclear Reactions 12 Grade (Physics SL)Document8 pagesScience Department: Topic 7.1 - Discrete Energy & Radioactivity Topic 7.2 - Nuclear Reactions 12 Grade (Physics SL)ananNo ratings yet
- Section A: Answer All Questions (40 Marks)Document11 pagesSection A: Answer All Questions (40 Marks)c3mutNo ratings yet
- Form 5 Science Term 1 Exam 24 With Ans KeyDocument12 pagesForm 5 Science Term 1 Exam 24 With Ans KeyAdrianNo ratings yet
- SHREE POKHARIYA SECONDARY SCHOOL Class 11 Tech.Document2 pagesSHREE POKHARIYA SECONDARY SCHOOL Class 11 Tech.pakheyyyNo ratings yet
- SAT Chemistry Eng-11-12 G AdvancedDocument80 pagesSAT Chemistry Eng-11-12 G AdvancedАрнур ОспановNo ratings yet
- SAT Chemistry Eng 11-12 G StandardDocument68 pagesSAT Chemistry Eng 11-12 G StandardAikerim BolysbekNo ratings yet
- Phy 441 Question BankDocument6 pagesPhy 441 Question Bankandrew silungweNo ratings yet
- Sup 2019Document1 pageSup 2019Prashanna YadavNo ratings yet
- PY2 P.test Stage 9 ScienceDocument260 pagesPY2 P.test Stage 9 Scienceguozi509509No ratings yet
- Chemistry SSC-I Slo Solution of 2nd Set Model Question PaperDocument10 pagesChemistry SSC-I Slo Solution of 2nd Set Model Question PaperHuzaifa NaeemNo ratings yet
- Chemistry 2023 Question PaperDocument7 pagesChemistry 2023 Question Papercabek22797No ratings yet
- 11 ChemistryDocument5 pages11 Chemistrykrishbhatia1503No ratings yet
- NuclearDocument8 pagesNuclearsnipersingh666No ratings yet
- Physics Sample Paper 2Document11 pagesPhysics Sample Paper 2Siddhi GoplanNo ratings yet
- ACIDS and BASES Notes & WorksheetDocument9 pagesACIDS and BASES Notes & WorksheetAdeenaNo ratings yet
- Formula of Sodium Chloride - Google SearchDocument1 pageFormula of Sodium Chloride - Google SearchEkta Mahila NTT Institute IwevsNo ratings yet
- Observation/ Problem/ Research Question Stated:: Sba # Yy MM DDDocument2 pagesObservation/ Problem/ Research Question Stated:: Sba # Yy MM DDRidhi ParwaniNo ratings yet
- 7 Eaf BofDocument28 pages7 Eaf BofMuhammad Umar Al FaruqNo ratings yet
- Chemistry Chapter 8 SaltsDocument32 pagesChemistry Chapter 8 SaltsnorlieyNo ratings yet
- Name ReactionsDocument10 pagesName ReactionsMUKUL SINGHNo ratings yet
- 1.1.1 Aluminium Tank AnodesDocument1 page1.1.1 Aluminium Tank AnodesSoltani AliNo ratings yet
- Aluminium SpecificationDocument2 pagesAluminium SpecificationkmrsekharNo ratings yet
- Metallurgy: - : General Principles and Processes of Isolation of Elements Class 12 Notes PDFDocument2 pagesMetallurgy: - : General Principles and Processes of Isolation of Elements Class 12 Notes PDFMahesh BabuNo ratings yet
- SSS 2 E-Note 1st Term ChemistryDocument23 pagesSSS 2 E-Note 1st Term ChemistryDave Blessed90% (10)
- CBSE Class 12 Question Paper Solution 2016 Chemistry Set 1Document5 pagesCBSE Class 12 Question Paper Solution 2016 Chemistry Set 1Savan PatelNo ratings yet
- Registered Pesticide 1 Oct 2007 - 1 Sept 2012Document312 pagesRegistered Pesticide 1 Oct 2007 - 1 Sept 2012marzuki2870% (1)
- Chemical Test Procedure FinalDocument51 pagesChemical Test Procedure Finallekshmi_remesh100% (2)
- Husn KimiaDocument10 pagesHusn KimiahusniNo ratings yet
- Isri Scrap Specifications Circular 2006 PDFDocument57 pagesIsri Scrap Specifications Circular 2006 PDFNazeeh Abdulrhman AlbokaryNo ratings yet
- Iron-Iron Carbide Phase Diagram: Effect of Pressure On Allotropy of IronDocument5 pagesIron-Iron Carbide Phase Diagram: Effect of Pressure On Allotropy of IronnareshNo ratings yet
- Preparation of SaltsDocument8 pagesPreparation of SaltsRose MusariraNo ratings yet
- PQR - 152Document3 pagesPQR - 152MAT-LIONNo ratings yet
- Calcium PreprationsDocument14 pagesCalcium PreprationsmtkapoorNo ratings yet
- Quantitative Analysis of A Sulfate: I. RationaleDocument5 pagesQuantitative Analysis of A Sulfate: I. RationaleBhupesh MulikNo ratings yet
- Stainless Steels & Nickel AlloysDocument108 pagesStainless Steels & Nickel AlloysAhmad Aloudah50% (8)
- Chem 2Document9 pagesChem 2Gajendra SinghNo ratings yet
- Sample Paper Science - 6 GurukulDocument8 pagesSample Paper Science - 6 GurukulGurukul PatnaNo ratings yet
- L1 NCERT P BLOCK GP 13Document48 pagesL1 NCERT P BLOCK GP 13bansalharshvardhan0No ratings yet
- Daily Welding Checklist (Sample)Document7 pagesDaily Welding Checklist (Sample)Siddiqui Abdul KhaliqNo ratings yet
- Clinker ReductionDocument2 pagesClinker ReductionGiequatNo ratings yet
- Basic Welding Filler Metal Technology: Lesson Ix Estimating and Comparing Weld Metal CostsDocument1 pageBasic Welding Filler Metal Technology: Lesson Ix Estimating and Comparing Weld Metal CostsRené Andrade MuñozNo ratings yet
- UntitledDocument14 pagesUntitledPacific ChemicalsNo ratings yet
- Heavy Hex Bolt DimensionsDocument6 pagesHeavy Hex Bolt DimensionsSUJIT PATEL100% (1)
- M/s Encorp Powertrans (P) LTDDocument3 pagesM/s Encorp Powertrans (P) LTDvijaymandiNo ratings yet