Professional Documents
Culture Documents
Free Fall - Definition, Examples, & Facts - Britannica
Uploaded by
reddygrOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Free Fall - Definition, Examples, & Facts - Britannica
Uploaded by
reddygrCopyright:
Available Formats
me Games & Quizzes History & Society Science & Tech Biographies Animals & Nature Geography
Home keyboard_arrow_right Science keyboard_arrow_right Physics keyboard_arrow_right Matter & Energy
Science & Tech
Brownian motion
physics
print Print verified Cite share Share message Feedback more_vert
Also known as: Brownian movement
Written and fact-checked by The Editors of Encyclopaedia Britannica
Last Updated: Mar 8, 2024 • Article History
toc Table of Contents
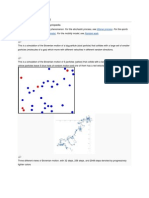
Brownian motion, any of various physical
phenomena in which some quantity is
constantly undergoing small, random
fluctuations. It was named for the Scottish
zoom_in
botanist Robert Brown, the first to study
such fluctuations (1827).
If a number of particles subject to Brownian
Brownian particle
motion are present in a given medium and
there is no preferred direction for the See all media
random oscillations, then over a period of
Category: Science & Tech
time the particles will tend to be spread
Also called: Brownian movement
evenly throughout the medium. Thus, if A
Key People: Albert Einstein • Robert
and B are two adjacent regions and, at time t,
Brown • Wendelin Werner • Jean
A contains twice as many particles as B, at Perrin
that instant the probability of a particle’s Related Topics: motion • kinetic
theory
leaving A to enter B is twice as great as the
On the Web: Massachusetts Institute
probability that a particle will leave B to
of Technology - Brownian Motion
enter A. The physical process in which a (Mar. 08, 2024)
substance tends to spread steadily from
regions of high concentration to regions of See all related content →
lower concentration is called diffusion.
Diffusion can therefore be considered a
macroscopic manifestation of Brownian motion on the microscopic level. Thus, it
is possible to study diffusion by simulating the motion of a Brownian particle and
computing its average behaviour. A few examples of the countless diffusion
processes that are studied in terms of Brownian motion include the diffusion of
pollutants through the atmosphere, the diffusion of “holes” (minute regions in
which the electrical charge potential is positive) through a semiconductor, and the
diffusion of calcium through bone tissue in living organisms.
Britannica Quiz
Physics and Natural Law
Early investigations
The term “classical Brownian motion” describes the random movement of
microscopic particles suspended in a liquid or gas. Brown was investigating the
fertilization process in Clarkia pulchella, then a newly discovered species of
flowering plant, when he noticed a “rapid oscillatory motion” of the microscopic
particles within the pollen grains suspended in water under the microscope. Other
researchers had noticed this phenomenon earlier, but Brown was the first to study
it. Initially he believed that such motion was a vital activity peculiar to the male sex
cells of plants, but he then checked to see if the pollen of plants dead for over a
century showed the same movement. Brown called this a “very unexpected fact of
seeming vitality being retained by these ‘molecules’ so long after the death of the
plant.” Further study revealed that the same motion could be observed not only
with particles of other organic substances but even with chips of glass or granite
and particles of smoke. Finally, in inarguable support of the nonliving nature of the
phenomenon, he demonstrated it in fluid-filled vesicles in rock from the Great
Sphinx.
Early explanations attributed the motion to thermal convection currents in the
fluid. When observation showed that nearby particles exhibited totally
uncorrelated activity, however, this simple explanation was abandoned. By the
1860s theoretical physicists had become interested in Brownian motion and were
searching for a consistent explanation of its various characteristics: a given particle
appeared equally likely to move in any direction; further motion seemed totally
unrelated to past motion; and the motion never stopped. An experiment (1865) in
which a suspension was sealed in glass for a year showed that the Brownian
motion persisted. More systematic investigation in 1889 determined that small
particle size and low viscosity of the surrounding fluid resulted in faster motion.
Einstein’s theory of Brownian motion
Since higher temperatures also led to more-rapid Brownian motion, in 1877 it was
suggested that its cause lay in the “thermal molecular motion in the liquid
environment.” The idea that molecules of a liquid or gas are constantly in motion,
colliding with each other and bouncing back and forth, is a prominent part of the
kinetic theory of gases developed in the third quarter of the 19th century by the
physicists James Clerk Maxwell, Ludwig Boltzmann, and Rudolf Clausius in
explanation of heat phenomena. According to the theory, the temperature of a
substance is proportional to the average kinetic energy with which the molecules of
the substance are moving or vibrating. It was natural to guess that somehow this
motion might be imparted to larger particles that could be observed under the
microscope; if true, this would be the first directly observable effect that would
corroborate the kinetic theory. This line of reasoning led the German physicist
Albert Einstein in 1905 to produce his quantitative theory of Brownian motion.
Similar studies were carried out on Brownian motion, independently and almost at
the same time, by the Polish physicist Marian Smoluchowski, who used methods
somewhat different from Einstein’s.
Einstein wrote later that his major aim
was to find facts that would guarantee as
much as possible the existence of atoms of
definite size. In the midst of this work, he play_arrow
discovered that according to atomic theory
there would have to be an observable
movement of suspended microscopic
Learn about Albert Einstein's
particles. Einstein did not realize that theory of Brownian motion and
observations concerning the Brownian how he derived the size of atoms
based on how much the Brownian
motion were already long familiar. particles move
Reasoning on the basis of statistical Description of Albert Einstein's theory of
Brownian motion and how he derived t…
...(more)
mechanics, he showed that for such a
See all videos for this article
microscopic particle the random
difference between the pressure of
molecular bombardment on two opposite sides would cause it to constantly wobble
back and forth. A smaller particle, a less viscous fluid, and a higher temperature
would each increase the amount of motion one could expect to observe. Over a
period of time, the particle would tend to drift from its starting point, and, on the
basis of kinetic theory, it is possible to compute the probability (P) of a particle’s
moving a certain distance (x) in any given direction (the total distance it moves will
be greater than x) during a certain time interval (t) in a medium whose coefficient
of diffusion (D) is known, D being equal to one-half the average of the square of
the displacement in the x-direction. This formula for probability “density” allows P
to be plotted against x. The graph is the familiar bell-shaped Gaussian “normal”
curve that typically arises when the random variable is the sum of many
independent, statistically identical random variables, in this case the many little
pushes that add up to the total motion. The equation for this relationship is
Get a Britannica Premium subscription and gain access to exclusive content.
Subscribe Now
The introduction of the ultramicroscope in 1903 aided quantitative studies by
making visible small colloidal particles whose greater activity could be measured
more easily. Several important measurements of this kind were made from 1905 to
1911. During this period the French physicist Jean-Baptiste Perrin was successful
in verifying Einstein’s analysis, and for this work he was awarded the Nobel Prize
for Physics in 1926. His work established the physical theory of Brownian motion
and ended the skepticism about the existence of atoms and molecules as actual
physical entities.
This article was most recently revised and updated by Erik Gregersen.
Home keyboard_arrow_right Science keyboard_arrow_right Physics keyboard_arrow_right Matter & Energy
Science & Tech
freefall
physics
print Print verified Cite share Share message Feedback more_vert
Written and fact-checked by The Editors of Encyclopaedia Britannica
Last Updated: Jan 30, 2024 • Article History
toc Table of Contents
Freefall, in mechanics, state of a body that
Category: Science & Tech
moves freely in any manner in the presence
Related Topics: gravity •
of gravity. The planets, for example, are in weightlessness • motion • equivalence
free fall in the gravitational field of the Sun. principle • gravitation
An astronaut orbiting Earth in a spacecraft
See all related content →
experiences a condition of weightlessness
because both the spacecraft and the
astronaut are in free fall. Both experience the
same gravitational pull from Earth, but the spacecraft does not ultimately fall to
the ground, because its forward velocity keeps it in orbit around Earth.
Gravitational forces are never uniform, and therefore only the centre of mass is in
free fall. All other points of a body are subject to tidal forces because they move in
a slightly different gravitational field. Earth is in free fall, but the pull of the Moon
is not the same at Earth’s surface as at its centre; the rise and fall of ocean tides
occur because the oceans are not in perfect free fall.
The Editors of Encyclopaedia Britannica
This article was most recently revised and updated by Erik Gregersen.
Home keyboard_arrow_right Science keyboard_arrow_right Mathematics
Science & Tech
Wendelin Werner
French mathematician
print Print verified Cite share Share message Feedback
Written by Jeremy John Gray
Fact-checked by The Editors of Encyclopaedia Britannica
Article History
toc Table of Contents
Wendelin Werner (born September 23,
Category: Science & Tech
1968, Cologne, West Germany [now in
Born: September 23, 1968, Cologne,
Germany]) German-born French West Germany [now in Germany] (age
mathematician who was awarded a Fields 55)
Medal in 2006 “for his contributions to the Awards And Honors: Fields Medal
(2006)
development of stochastic Loewner
Subjects Of Study: Brownian motion
evolution, the geometry of two-dimensional
Brownian motion, and conformal theory.”
See all related content →
Werner received a doctorate from the
University of Paris VI (1993). In 1997 he
became a professor of mathematics at the University of Paris-Sud in Orsay, and he
held that post until 2013, when he joined the faculty at ETH Zürich.
Load Next Page
keyboard_arrow_down
Britannica Quiz
Numbers and Mathematics
Brownian motion is the best-understood mathematical model of diffusion and is
applicable in a wide variety of cases, such as the seepage of water or pollutants
through rock. It is often invoked in the study of phase transitions, such as the
freezing or boiling of water, in which the system undergoes what are called critical
phenomena and becomes random at any scale. In 1982 the American physicist
Kenneth G. Wilson received a Nobel Prize for his investigations into a seemingly
universal property of physical systems near critical points, expressed as a power
law and determined by the qualitative nature of the system and not its microscopic
properties. In the 1990s, Wilson’s work was extended to the domain of conformal
field theory, which relates to the string theory of fundamental particles. Rigorous
theorems and geometrical insight, however, were lacking until the work of Werner
and his collaborators gave the first picture of systems at and near their critical
points.
Werner also verified a 1982 conjecture by the Polish mathematician Benoit
Mandelbrot that the boundary of a random walk in the plane (a model for the
diffusion of a molecule in a gas) has a fractal dimension of 4/ (between a one-
3
dimensional line and a two-dimensional plane). Werner also showed that there is a
self-similarity property for these walks that derives from a property, only
conjectural until his work, that various aspects of Brownian motion are
conformally invariant. His other awards included a European Mathematical
Society Prize (2000) and a Fermat Prize (2001).
Jeremy John Gray
You might also like
- 2018 Grade 10 Chemistry Notes PDFDocument106 pages2018 Grade 10 Chemistry Notes PDFHon Oscar Zulu87% (23)
- HND 1 Electrical Measurement and Control EEI 311 2016Document2 pagesHND 1 Electrical Measurement and Control EEI 311 2016iskeel75% (4)
- David Bohm's Theory of the Implicate OrderDocument5 pagesDavid Bohm's Theory of the Implicate OrderHaris_IsaNo ratings yet
- Karthikeya XII-A Physics Project PDFDocument23 pagesKarthikeya XII-A Physics Project PDFkarthikeya kakarlapudiNo ratings yet
- Brownian MotionDocument31 pagesBrownian Motionsujatha ramkiNo ratings yet
- 08 SampMathDocument1 page08 SampMathShaun MilesNo ratings yet
- Brownian MotionDocument25 pagesBrownian Motionsujatha ramkiNo ratings yet
- 0063 0078Document16 pages0063 0078Anurag AnandNo ratings yet
- Brownian Motion SimulationDocument21 pagesBrownian Motion SimulationrayroyNo ratings yet
- Soft Matter: Tutorial ReviewDocument16 pagesSoft Matter: Tutorial ReviewrevNo ratings yet
- An Introduction To Fractional Diffusion PDFDocument54 pagesAn Introduction To Fractional Diffusion PDFChế LinhNo ratings yet
- 2005 Frey Brownian Motion A Paradigm of Soft Matter and Biological PhysicsDocument31 pages2005 Frey Brownian Motion A Paradigm of Soft Matter and Biological PhysicscsNo ratings yet
- Superluminals and The Speed of LightDocument5 pagesSuperluminals and The Speed of LightMia AmaliaNo ratings yet
- Is The Moon There When Nobody Looks MERMINDocument11 pagesIs The Moon There When Nobody Looks MERMINbob n sauveNo ratings yet
- Universe Past PresentDocument4 pagesUniverse Past PresentSamay katariaNo ratings yet
- The Quantum Leap: From Uncertainty to EntanglementDocument13 pagesThe Quantum Leap: From Uncertainty to EntanglementMuhammad Fahim HasanNo ratings yet
- Reaction PaperDocument2 pagesReaction PaperVasquez Austine ThriceNo ratings yet
- Explaining Michelson-Morley without Special RelativityDocument3 pagesExplaining Michelson-Morley without Special RelativityAndré AbramczukNo ratings yet
- TeleportationDocument4 pagesTeleportationDeepankar Anil KumarNo ratings yet
- MazoDocument4 pagesMazomarcoNo ratings yet
- Quantum Gravity Theory Explains ConsciousnessDocument16 pagesQuantum Gravity Theory Explains ConsciousnessJorgee_Porgee_8203No ratings yet
- Vortex Formation in The Wake of Dark Matter PropulDocument15 pagesVortex Formation in The Wake of Dark Matter Propulfernando alves ferreiraNo ratings yet
- Brownian MotionDocument76 pagesBrownian MotionPraveen KumarNo ratings yet
- Random Vibration - A Brief History: Thomas L. Paez, Thomas Paez Consulting, Durango ColoradoDocument9 pagesRandom Vibration - A Brief History: Thomas L. Paez, Thomas Paez Consulting, Durango ColoradojorgeNo ratings yet
- Big Bang Scientific Religious DiscussionsDocument4 pagesBig Bang Scientific Religious DiscussionsHagoub ArteenNo ratings yet
- The Labyrinth of Quantum LogicDocument41 pagesThe Labyrinth of Quantum LogicJordan BlevinsNo ratings yet
- Brownian Poster FinalDocument1 pageBrownian Poster Finalbecheanu13No ratings yet
- Free download Physics 100 Ideas In 100 Words 1St Edition David Sang full chapter pdf epubDocument51 pagesFree download Physics 100 Ideas In 100 Words 1St Edition David Sang full chapter pdf epubroger.leavell785100% (11)
- The Principle of Uncertainty - GamowDocument9 pagesThe Principle of Uncertainty - GamowPPAANo ratings yet
- Superluminals and the Speed of Light DebateDocument5 pagesSuperluminals and the Speed of Light DebateSarbu AnaNo ratings yet
- The Mystery of MatterDocument86 pagesThe Mystery of MattercumdjafNo ratings yet
- Mechanical Explanations of GravitationDocument7 pagesMechanical Explanations of GravitationtechzonesNo ratings yet
- Quantum Theory and the Role of Mind in NatureDocument42 pagesQuantum Theory and the Role of Mind in NatureEyiogbeNo ratings yet
- Candidate No: 3083Document7 pagesCandidate No: 3083Afzal HussainNo ratings yet
- GravitationDocument16 pagesGravitationGraecus1No ratings yet
- Fatematun Nur Tofa (20201128)Document1 pageFatematun Nur Tofa (20201128)Ariful Hassan SaikatNo ratings yet
- Einstein & Brownian Motion - Expanding The Atomic Theory - Risin Expert SessionDocument8 pagesEinstein & Brownian Motion - Expanding The Atomic Theory - Risin Expert SessionHR RisinNo ratings yet
- Big Bang ClockDocument8 pagesBig Bang Clockdeep mitraNo ratings yet
- Bush PHYSICS TODAY2015 PDFDocument9 pagesBush PHYSICS TODAY2015 PDFAyussh VoltegourdeNo ratings yet
- (Ebook) Radionics - Opening Up The Black Box-1Document6 pages(Ebook) Radionics - Opening Up The Black Box-1victoriacharcasNo ratings yet
- The Big Bang Theory ExplainedDocument3 pagesThe Big Bang Theory ExplainedEunice Acuna100% (1)
- Quantum 'Spooky Action at A Distance' Travels at Least 10,000 Times Faster Than LightDocument2 pagesQuantum 'Spooky Action at A Distance' Travels at Least 10,000 Times Faster Than LightquantumrealmNo ratings yet
- Black Hole, White Hole, Wormhole and CPH TheoryDocument18 pagesBlack Hole, White Hole, Wormhole and CPH TheoryNandita BalajiNo ratings yet
- 020 FTLinPrespacetime PSTJ113May201658 4961 1 PBDocument24 pages020 FTLinPrespacetime PSTJ113May201658 4961 1 PBGavrila LucianNo ratings yet
- Basic Quantum TheoryDocument3 pagesBasic Quantum Theoryrahsan.swrNo ratings yet
- QM - NotesDocument14 pagesQM - NotesShubham MishraNo ratings yet
- Big Bang 2 PDFDocument15 pagesBig Bang 2 PDFJoseNo ratings yet
- Uncertainty Einstein, Heisenberg, Bohr, and The Struggle For The Soul of Science by David Lindley Richard A Blasband (Vol 18 No 2)Document10 pagesUncertainty Einstein, Heisenberg, Bohr, and The Struggle For The Soul of Science by David Lindley Richard A Blasband (Vol 18 No 2)Cambiador de MundoNo ratings yet
- Wormholes and the Paranormal Event HorizonDocument18 pagesWormholes and the Paranormal Event HorizonAbdullah DiefNo ratings yet
- Isaac Newton ThesisDocument7 pagesIsaac Newton Thesiscdayxnzcf100% (2)
- Wave-Particle Duality FundamentalsDocument7 pagesWave-Particle Duality FundamentalsRahmatullahNo ratings yet
- Radionicsblackbox PDFDocument6 pagesRadionicsblackbox PDFStellaEstelNo ratings yet
- Bush Physics Today2015Document8 pagesBush Physics Today2015TomCafezNo ratings yet
- Dark MatterDocument5 pagesDark MatterTashiNo ratings yet
- Realist AnalysisDocument19 pagesRealist AnalysisLe Thu ThaoNo ratings yet
- Eva Bagona's Reaction to Gravitational Lensing and Black HolesDocument6 pagesEva Bagona's Reaction to Gravitational Lensing and Black HolesGrace SamNo ratings yet
- 2 Special Rel.Document34 pages2 Special Rel.JoseNo ratings yet
- The Origin of The Universe and The Solar SystemDocument7 pagesThe Origin of The Universe and The Solar SystemThea Aaliyah Cruz ShalimNo ratings yet
- Theory of RelativityDocument2 pagesTheory of Relativitykrishna sharmaNo ratings yet
- Dilation Flooding: A Solution For Cosmic Redshift Based On Gravity Wave PropagationFrom EverandDilation Flooding: A Solution For Cosmic Redshift Based On Gravity Wave PropagationNo ratings yet
- The Changing Conceptions of the Universe - From Newton to Einstein -From EverandThe Changing Conceptions of the Universe - From Newton to Einstein -No ratings yet
- 18 G of Glucose, C6H12O6, Is Dissolved in 1 KG of WaterDocument1 page18 G of Glucose, C6H12O6, Is Dissolved in 1 KG of WaterreddygrNo ratings yet
- A Cell When Dipped in 0.5 M Sucrose Solution HaDocument2 pagesA Cell When Dipped in 0.5 M Sucrose Solution HareddygrNo ratings yet
- The Van't Hoff Factor I' ForDocument2 pagesThe Van't Hoff Factor I' ForreddygrNo ratings yet
- MCQ's On Chemistry in Everyday LifeDocument5 pagesMCQ's On Chemistry in Everyday LifereddygrNo ratings yet
- Surface Chemistry - Practically Study MaterialDocument20 pagesSurface Chemistry - Practically Study MaterialreddygrNo ratings yet
- Business Structure: LLP Company Registration: Compile Financial Estimates. Have To Work On Regular BasisDocument4 pagesBusiness Structure: LLP Company Registration: Compile Financial Estimates. Have To Work On Regular BasisreddygrNo ratings yet
- Semiconductors and DopingDocument3 pagesSemiconductors and DopingreddygrNo ratings yet
- Which Has The Highest Dipole Moment-2Document3 pagesWhich Has The Highest Dipole Moment-2reddygrNo ratings yet
- Hydrogen SulfideDocument151 pagesHydrogen SulfidereddygrNo ratings yet
- What Is Doping - What Are N-Type and P-Type SemiconductorsDocument1 pageWhat Is Doping - What Are N-Type and P-Type SemiconductorsreddygrNo ratings yet
- Class 12th Chemistry Haloalkanes and Haloarenes NCERT Notes CBSE 2023Document4 pagesClass 12th Chemistry Haloalkanes and Haloarenes NCERT Notes CBSE 2023reddygrNo ratings yet
- Periodic Table With Element NamesDocument3 pagesPeriodic Table With Element NamesreddygrNo ratings yet
- Halogens - Periodic Table - ChemTalkDocument4 pagesHalogens - Periodic Table - ChemTalkreddygrNo ratings yet
- Racemic ModificationDocument32 pagesRacemic ModificationreddygrNo ratings yet
- NounsDocument24 pagesNounsBharath Kumar BKNo ratings yet
- SRM-AP SEAS-Brochure 2022Document2 pagesSRM-AP SEAS-Brochure 2022reddygrNo ratings yet
- Group 17 General Properties of HalogensDocument8 pagesGroup 17 General Properties of HalogensreddygrNo ratings yet
- How To Calculate The Degree of PolymerizationDocument2 pagesHow To Calculate The Degree of PolymerizationreddygrNo ratings yet
- Full Breath Retention - Kumbhaka PranayamaDocument6 pagesFull Breath Retention - Kumbhaka PranayamareddygrNo ratings yet
- Business Structure: LLP Company Registration: Compile Financial Estimates. Have To Work On Regular BasisDocument4 pagesBusiness Structure: LLP Company Registration: Compile Financial Estimates. Have To Work On Regular BasisreddygrNo ratings yet
- Reference 09 10 2017 205905Document64 pagesReference 09 10 2017 205905reddygrNo ratings yet
- HealthDocument21 pagesHealthreddygrNo ratings yet
- Full Breath Retention - Kumbhaka PranayamaDocument6 pagesFull Breath Retention - Kumbhaka PranayamareddygrNo ratings yet
- IR Shifts of Carbonyl GroupsDocument2 pagesIR Shifts of Carbonyl GroupsreddygrNo ratings yet
- Synthesis of Sulphonated CalixiranesDocument6 pagesSynthesis of Sulphonated CalixiranesreddygrNo ratings yet
- Reference 09 10 2017 205905Document64 pagesReference 09 10 2017 205905reddygrNo ratings yet
- Asian Age 03.08.17Document16 pagesAsian Age 03.08.17reddygrNo ratings yet
- National Flag & SongDocument33 pagesNational Flag & SongreddygrNo ratings yet
- సంజీవరాయ శర్మDocument2 pagesసంజీవరాయ శర్మreddygrNo ratings yet
- Structural Analysis and Design of A Three-Storey Reinforced Concrete Design of School BuildingDocument51 pagesStructural Analysis and Design of A Three-Storey Reinforced Concrete Design of School BuildingVenus Gjervie Yu - Alvarez100% (1)
- Molecular Spectroscopy MCQsDocument19 pagesMolecular Spectroscopy MCQsManikandan KNo ratings yet
- Current Concepts: Biomechanics of Knee LigamentsDocument11 pagesCurrent Concepts: Biomechanics of Knee LigamentsBarbaraAndradeQuirozNo ratings yet
- FlenderTechnicalHandbook PDFDocument79 pagesFlenderTechnicalHandbook PDFWagner OliveiraNo ratings yet
- 09-25 Problem SetDocument2 pages09-25 Problem SetsonjabottlelawNo ratings yet
- Essar Sample QuestionsDocument3 pagesEssar Sample Questionshgbv tttbNo ratings yet
- For StudentsDocument82 pagesFor StudentsFaisal Mumtaz100% (1)
- Section Study Guide: Teacher Notes and AnswersDocument5 pagesSection Study Guide: Teacher Notes and AnswerszahraNo ratings yet
- RF4 PK PDFDocument2 pagesRF4 PK PDFMuhammad WaseemNo ratings yet
- Dynamic Modeling of Compressors Illustrated by An Oil Flooded Twin Helical Screw Compressor 2011 MechatronicsDocument8 pagesDynamic Modeling of Compressors Illustrated by An Oil Flooded Twin Helical Screw Compressor 2011 MechatronicsTai Huu100% (1)
- CH 10Document76 pagesCH 10Jason Enduro BayuNo ratings yet
- Unit 6Document10 pagesUnit 6Hussain GhaziNo ratings yet
- BHMY 2x1 Flowgrid SP FS 34016A 1120 EnglishDocument2 pagesBHMY 2x1 Flowgrid SP FS 34016A 1120 EnglishLuis Alberto Portugal MariacalNo ratings yet
- D PHYSICS - TOP 40 QUESTIONS FOR EXAMSDocument27 pagesD PHYSICS - TOP 40 QUESTIONS FOR EXAMSSaheli DeyNo ratings yet
- Cheat Sheet SurfaceDocument2 pagesCheat Sheet SurfaceArchita VNo ratings yet
- PT-Liquid Penetrant Testing Procedure R01 - 2 2Document12 pagesPT-Liquid Penetrant Testing Procedure R01 - 2 2George Ogbeche100% (1)
- Massachusetts Institute of Technology Department of Electrical Engineering and Computer ScienceDocument3 pagesMassachusetts Institute of Technology Department of Electrical Engineering and Computer ScienceKrishna GNo ratings yet
- 7673 - 18596B-C Tray Arm AssemblyDocument2 pages7673 - 18596B-C Tray Arm AssemblyGabrielNo ratings yet
- Cilamce 2013 - 0690Document20 pagesCilamce 2013 - 0690José Guilherme Santos da SilvaNo ratings yet
- Diesel engine heat balance sheetDocument2 pagesDiesel engine heat balance sheetsamarthgkNo ratings yet
- Mechanisms of Nucleophilic Substitution ReactionsDocument16 pagesMechanisms of Nucleophilic Substitution ReactionsFachmy Hamdani100% (1)
- Lectures 6: Absorption in Tray TowerDocument11 pagesLectures 6: Absorption in Tray TowerMihir Kumar MechNo ratings yet
- S861 & S866 Alfa LavalDocument176 pagesS861 & S866 Alfa LavalarmaganNo ratings yet
- Homework 6Document5 pagesHomework 6Stephen RandallNo ratings yet
- 14 - Microscope Parts - Powerpoint FreeDocument30 pages14 - Microscope Parts - Powerpoint Freeapi-340419341No ratings yet
- UAM Free Fall and Projectile MotionDocument64 pagesUAM Free Fall and Projectile MotionnoeldbelovedNo ratings yet
- Draft Specification of Various Items of TunnelsDocument29 pagesDraft Specification of Various Items of TunnelsDEBASIS BARMANNo ratings yet
- PV Elite: Input Echo, Leg & Lug Item 1, Description: Trunnion 1-2Document15 pagesPV Elite: Input Echo, Leg & Lug Item 1, Description: Trunnion 1-2SYedZYnAleNo ratings yet